Effects of Serum From Radiofrequency Ablation Patients Receiving General Anesthesia or Local Anesthesia on Hepatocellular Carcinoma Cancer Cell Malignancy: A Prospective Randomized Controlled Trial
- PMID: 34631520
- PMCID: PMC8495259
- DOI: 10.3389/fonc.2021.686294
Effects of Serum From Radiofrequency Ablation Patients Receiving General Anesthesia or Local Anesthesia on Hepatocellular Carcinoma Cancer Cell Malignancy: A Prospective Randomized Controlled Trial
Abstract
Background: Whether anesthesia methods affect malignant biological behavior of cancer remains unresolved. In this study, we aim to compare the effects of general anesthesia (GA) and local anesthesia (LA) on serum collected from primary hepatocellular carcinoma (HCC) patients presenting for radiofrequency ablation (RFA).
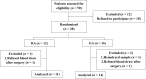
Methods: From August 2020 to December 2020, a prospective, randomized, and controlled study was conducted at Renji Hospital, which is affiliated with Shanghai Jiaotong University School of Medicine. 25 qualified patients from 18 to 65 years of age undergoing RFA were enrolled in the study and randomly assigned into two groups: the GA group (n = 14) and the LA group (n = 11). Venous blood was drawn from all patients preoperatively and 1 hour postoperatively. The serum collected was then used for the culturing of HepG2 cells. The malignant biological behaviors of HepG2 cells, including invasion, migration and proliferation, were observed after 24 hours of exposure to patients' serum. ELISA was used to compare expression levels of pro-inflammatory cytokines (IL-1β, IL-6, TNF-α) and lymphokines (IFN-γ, IL-2) in patients' serum from both groups.
Results: HepG2 cells cultured with postoperative serum obtained from patients who received GA, but not LA, were associated with significantly increased cell invasion, migration and proliferation, compared to preoperative serum from the same patient group. Expression levels of pro-inflammatory cytokines were significantly higher, and lymphokines significantly lower in postoperative serum from GA patients compared to the corresponding preoperative serum.
Conclusion: GA affects the serum milieu of patients with HCC, promoting the malignant biological behavior of a human HCC cell line.
Keywords: cancer cell malignancy; general anesthesia; hepatocellular carcinoma; local anesthesia; serum milieu.
Copyright © 2021 Shi, Wu, Wang, Liu, Wang, Luo, Su, Zhai and Tian.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
[Examination of the effect of anesthesia on radiofrequency ablation of hepatocellular carcinoma-a patient survey on anesthesia for radiofrequency ablation].Gan To Kagaku Ryoho. 2012 Nov;39(12):1843-5. Gan To Kagaku Ryoho. 2012. PMID: 23267905 Japanese.
-
Effects of general anesthesia versus local anesthesia in primary hepatocellular carcinoma patients presenting for thermal ablation surgery: a multiple center retrospective cohort study with propensity score matching.Ann Transl Med. 2020 Mar;8(6):277. doi: 10.21037/atm.2020.03.88. Ann Transl Med. 2020. PMID: 32355721 Free PMC article.
-
MR-guided microwave ablation of hepatocellular carcinoma (HCC): is general anesthesia more effective than local anesthesia?BMC Cancer. 2021 May 17;21(1):562. doi: 10.1186/s12885-021-08298-2. BMC Cancer. 2021. PMID: 34001036 Free PMC article.
-
General versus local anesthesia for percutaneous radiofrequency ablation of hepatocellular carcinoma at unusual regions.J Cancer Res Ther. 2020;16(7):1686-1690. doi: 10.4103/jcrt.JCRT_1187_20. J Cancer Res Ther. 2020. PMID: 33565517
-
Evaluation of clinical outcomes of radiofrequency ablation and surgical resection for hepatocellular carcinoma conforming to the Milan criteria: A systematic review and meta-analysis of recent randomized controlled trials.J Gastroenterol Hepatol. 2021 Jul;36(7):1769-1777. doi: 10.1111/jgh.15440. Epub 2021 Feb 25. J Gastroenterol Hepatol. 2021. PMID: 33569810
Cited by
-
Inhibition of SREBP-1 Activation by a Novel Small-Molecule Inhibitor Enhances the Sensitivity of Hepatocellular Carcinoma Tissue to Radiofrequency Ablation.Front Oncol. 2021 Nov 26;11:796152. doi: 10.3389/fonc.2021.796152. eCollection 2021. Front Oncol. 2021. PMID: 34900747 Free PMC article.
References
-
- Allemani C, Matsuda T, Di Carlo V, Harewood R, Matz M, Nikšić M, et al. . Global Surveillance of Trends in Cancer Survival 2000-14 (CONCORD-3): Analysis of Individual Records for 37 513 025 Patients Diagnosed With One of 18 Cancers From 322 Population-Based Registries in 71 Countries. Lancet (2018) 391(10125):1023–75. doi: 10.1016/s0140-6736(17)33326-3 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

