HPV Positive Status Is a Favorable Prognostic Factor in Non-Nasopharyngeal Head and Neck Squamous Cell Carcinoma Patients: A Retrospective Study From the Surveillance, Epidemiology, and End Results Database
- PMID: 34631523
- PMCID: PMC8497986
- DOI: 10.3389/fonc.2021.688615
HPV Positive Status Is a Favorable Prognostic Factor in Non-Nasopharyngeal Head and Neck Squamous Cell Carcinoma Patients: A Retrospective Study From the Surveillance, Epidemiology, and End Results Database
Abstract
Objective: To investigate the impact of the human papillomavirus (HPV) status on head and neck squamous cell carcinoma (HNSCC) arising from different anatomic subsites.
Methods: HNSCC patients with known HPV status from the Surveillance, Epidemiology, and End Results (SEER) database between 2010-2015 were included in our analysis. Patients were classified into three categories of HNSCC according to Site recode ICD-O-3/WHO 2008 and Primary Site-labeled, namely, oropharynx, hypopharynx, and nasopharynx. Logistic regression model was conducted to evaluate the relationship between patient characteristics and HPV status. Kaplan-Meier methods and COX regression analysis were used to analyze survival data.
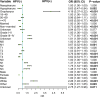
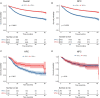
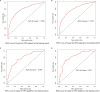
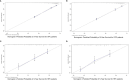
Results: A total of 9,943 HNSCC patients with known HPV status from the SEER database were enrolled, with 6,829 (68.7%) HPV-positive patients. HPV-positive and HPV-negative HNSCC were distinct and had different clinical and socioeconomic features (all P < 0.001). Primary sites, socioeconomical factors (age, sex, marital status, and race), and pathological features (TNM stage and grade) were closely related with HPV status (all P < 0.001). HPV-positive status was a favorable prognostic marker in HNSCC patients with cancers of the oropharynx and hypopharynx (all P < 0.001), but was not in nasopharyngeal carcinoma patients (P = 0.843). A total of 8,933 oropharyngeal carcinoma (OPC) and 558 hypopharyngeal carcinoma (HPC) patients were divided into the training and validation cohorts with a ratio of 1:1. Significant prognostic factors of the OS yielded by multivariate COX analysis in the training cohort were integrated to construct nomograms for OPC and HPC patients. The prognostic models showed a good discrimination with a C-index of 0.79 ± 0.007 and 0.73 ± 0.023 in OPC and HPC, respectively. Favorable calibration was reflected by the calibration curves. Additionally, corresponding risk classification systems for OPC and HPC patients based on the nomograms were built and could perfectly classify patients into low-risk, intermediated-risk, high-risk groups. OS in the three risk groups was accurately differentiated and showed a good discrimination.
Conclusion: HPV positivity was associated with an improved survival in HNSCC patients with cancers of the oropharynx and hypopharynx. Nomograms and corresponding risk classification systems were constructed to assist clinicians in evaluating the survival of OPC and HPC patients.
Keywords: SEER database; head and neck squamous cell carcinoma (HNSCC); human papillomavirus (HPV); nomogram; prognosis.
Copyright © 2021 Wu, Wang, Liu, Wang, Li, Hu, Qiu, Liang, Wei and Zhong.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
A novel nomogram and risk classification system for predicting overall survival in head and neck squamous cell cancer with distant metastasis at initial diagnosis.Eur Arch Otorhinolaryngol. 2023 Mar;280(3):1467-1478. doi: 10.1007/s00405-022-07716-w. Epub 2022 Oct 31. Eur Arch Otorhinolaryngol. 2023. PMID: 36316576 Clinical Trial.
-
The prognosis of HPV-associated metastatic pharyngeal patients by primary and distant site.Oral Oncol. 2022 Feb;125:105675. doi: 10.1016/j.oraloncology.2021.105675. Epub 2021 Dec 27. Oral Oncol. 2022. PMID: 34968864 Free PMC article.
-
Association of Human Papillomavirus Status at Head and Neck Carcinoma Subsites With Overall Survival.JAMA Otolaryngol Head Neck Surg. 2018 Jun 1;144(6):519-525. doi: 10.1001/jamaoto.2018.0395. JAMA Otolaryngol Head Neck Surg. 2018. PMID: 29801040 Free PMC article.
-
Emerging insights into recurrent and metastatic human papillomavirus-related oropharyngeal squamous cell carcinoma.Laryngoscope Investig Otolaryngol. 2017 Jan 17;2(1):10-18. doi: 10.1002/lio2.37. eCollection 2017 Feb. Laryngoscope Investig Otolaryngol. 2017. PMID: 28894817 Free PMC article. Review.
-
The molecular mechanism of human papillomavirus-induced carcinogenesis in head and neck squamous cell carcinoma.Int J Clin Oncol. 2016 Oct;21(5):819-826. doi: 10.1007/s10147-016-1005-x. Epub 2016 Jun 23. Int J Clin Oncol. 2016. PMID: 27339270 Review.
Cited by
-
Distinct sociodemographic differences in incidence and survival rates for human papillomavirus (HPV)-like, non-HPV-like, and "other"-like oral cavity and pharynx cancers: An analysis of Surveillance, Epidemiology and End Results (SEER) Program data.Front Oncol. 2022 Aug 18;12:980900. doi: 10.3389/fonc.2022.980900. eCollection 2022. Front Oncol. 2022. PMID: 36072808 Free PMC article.
-
Human papillomavirus infection and non-oropharyngeal head and neck cancers: an umbrella review of meta-analysis.Eur Arch Otorhinolaryngol. 2023 Sep;280(9):3921-3930. doi: 10.1007/s00405-023-08027-4. Epub 2023 May 22. Eur Arch Otorhinolaryngol. 2023. PMID: 37212863
-
Increased Abundances of CD16+ Non-Classical Monocytes Accompany with Elevated Monocytic PD-L1 and CD4+ T Cell Disturbances in Oropharyngeal Cancer.Biomedicines. 2022 Jun 9;10(6):1363. doi: 10.3390/biomedicines10061363. Biomedicines. 2022. PMID: 35740384 Free PMC article.
-
Human papillomavirus-associated head and neck squamous cell carcinoma cells lose viability during triggered myocyte lineage differentiation.Cell Death Dis. 2024 Jul 19;15(7):517. doi: 10.1038/s41419-024-06867-4. Cell Death Dis. 2024. PMID: 39030166 Free PMC article.
-
The Role of the p16 and p53 Tumor Suppressor Proteins and Viral HPV16 E6 and E7 Oncoproteins in the Assessment of Survival in Patients with Head and Neck Cancers Associated with Human Papillomavirus Infections.Cancers (Basel). 2023 May 11;15(10):2722. doi: 10.3390/cancers15102722. Cancers (Basel). 2023. PMID: 37345059 Free PMC article.
References
-
- Cohen EEW, Bell RB, Bifulco CB, Burtness B, Gillison ML, Harrington KJ, et al. . The Society for Immunotherapy of Cancer Consensus Statement on Immunotherapy for the Treatment of Squamous Cell Carcinoma of the Head and Neck (HNSCC). J Immunother Cancer (2019) 7(1):184. doi: 10.1186/s40425-019-0662-5 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

