Case Report: Off-Label Use of Omalizumab in a 6-Year-Old Boy With ASD Ameliorated Severe Allergic Rhinitis and Subsequently Improved Behavioral Symptoms
- PMID: 34631617
- PMCID: PMC8493867
- DOI: 10.3389/fped.2021.714111
Case Report: Off-Label Use of Omalizumab in a 6-Year-Old Boy With ASD Ameliorated Severe Allergic Rhinitis and Subsequently Improved Behavioral Symptoms
Abstract
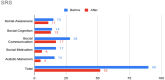
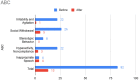
Children with ASD have elevated risk for developing allergic symptoms. The severity of allergic symptoms can exacerbate behavioral problems in children with ASD. Omalizumab, an anti-IgE antibody, has previously shown efficacy in treating allergic rhinitis and behavioral problems in a 12-year-old child with ASD. The present case report provides robust characterization of behavioral improvement in a 6-year-old child with ASD, allergic rhinitis, and autoimmune disorder. A 6-year-old boy with ASD and Hashimoto's disease presented to the clinic with severe allergic rhinitis, irritability, and language delay. After other treatments failed to improve symptoms, our patient was treated with omalizumab at 300 mg/month via subcutaneous injection for a total of 6 months. Marked improvement in allergic symptoms were observed at 2 months into treatment and were maintained through the treatment period. At the conclusion of the treatment period, results from multiple behavioral questionnaires, including the SRS-2, ABC, RBS-R, and PSQI, demonstrated substantial improvement in ASD-related behavioral symptoms. In this case, omalizumab markedly improved ASD-related and sleep behavior in a 6-year-old with ASD, allergic rhinitis, and autoimmune disorder. Future studies with larger patient populations are warranted to investigate the efficacy of omalizumab in patients with ASD and allergy symptoms.
Keywords: allergic rhinitis; anxiety; autism spectrum disorder; omalizumab; sleep disturbance.
Copyright © 2021 Kong, Clairmont and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Long-term omalizumab efficacy in allergic rhinitis.Immunol Lett. 2020 Nov;227:81-87. doi: 10.1016/j.imlet.2020.08.002. Epub 2020 Aug 13. Immunol Lett. 2020. PMID: 32798500
-
Effect of omalizumab on symptoms of seasonal allergic rhinitis: a randomized controlled trial.JAMA. 2001 Dec 19;286(23):2956-67. doi: 10.1001/jama.286.23.2956. JAMA. 2001. PMID: 11743836 Clinical Trial.
-
Effect of Omalizumab on Nasal Symptoms in Asthma and Perennial Allergic Rhinitis.J Coll Physicians Surg Pak. 2020 Nov;30(11):1170-1174. doi: 10.29271/jcpsp.2020.11.1170. J Coll Physicians Surg Pak. 2020. PMID: 33222734
-
Etiopathogenesis and management of perennial allergic rhinitis: a state-of-the-art review.Treat Respir Med. 2004;3(1):45-57. doi: 10.2165/00151829-200403010-00006. Treat Respir Med. 2004. PMID: 15174893 Review.
-
Anti-IgE treatment in allergic rhinitis.Int J Pediatr Otorhinolaryngol. 2019 Dec;127:109674. doi: 10.1016/j.ijporl.2019.109674. Epub 2019 Sep 10. Int J Pediatr Otorhinolaryngol. 2019. PMID: 31526939 Review.
Cited by
-
An Overview of Off-Label Use of Humanized Monoclonal Antibodies in Paediatrics.Medicina (Kaunas). 2022 Apr 29;58(5):625. doi: 10.3390/medicina58050625. Medicina (Kaunas). 2022. PMID: 35630042 Free PMC article. Review.
References
-
- Antony MM, Cox BJ, Enns MW, Bieling PJ, Swinson RP. Psychometric properties of the 42-item and 21-item versions of the depression anxiety stress scales in clinical groups and a community sample. Psychol Assess. (1998) 10:176–81. 10.1037/1040-3590.10.2.176 - DOI
-
- Ainsworth K, Robertson AE, Welsh H, Day M, Watt J, Barry F, et al. . Anxiety in adults with autism: Perspectives from practitioners. Res Autism Spec Dis. (2020) 69:101457. 10.1016/j.rasd.2019.101457 - DOI
Publication types
LinkOut - more resources
Full Text Sources

