Evaluation of Placental Transfer and Tissue Distribution of cis- and Trans-Permethrin in Pregnant Rats and Fetuses Using a Physiological-Based Pharmacokinetic Model
- PMID: 34631627
- PMCID: PMC8495120
- DOI: 10.3389/fped.2021.730383
Evaluation of Placental Transfer and Tissue Distribution of cis- and Trans-Permethrin in Pregnant Rats and Fetuses Using a Physiological-Based Pharmacokinetic Model
Abstract
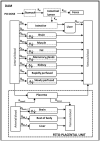
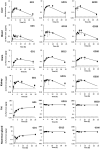
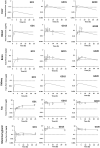
Biomonitoring studies have highlighted the exposure of pregnant women to pyrethroids based on the measurement of their metabolites in urine. Pyrethroids can cross the placental barrier and be distributed in the fetus as some pyrethroids were also measured in the meconium of newborns. Prenatal exposure to pyrethroids is suspected to alter the neurodevelopment of children, and animal studies have shown that early life exposure to permethrin, one of the most commonly used pyrethroid in household applications, can alter the brain development. This study aimed to characterize the fetal permethrin exposure throughout gestation in rats. We developed a pregnancy physiologically based pharmacokinetic (pPBPK) model that describes the maternal and fetal kinetics of the cis- and trans- isomers of permethrin during the whole gestation period. Pregnant Sprague-Dawley rats were exposed daily to permethrin (50 mg/kg) by oral route from the start of gestation to day 20. Permethrin isomers were quantified in the feces, kidney, mammary gland, fat, and placenta in dams and in both maternal and fetal blood, brain, and liver. Cis- and trans-permethrin were quantified in fetal blood and tissues, with higher concentrations for the cis-isomer. The pPBPK model was fitted to the toxicokinetic maternal and fetal data in a Bayesian framework. Several parameters were adjusted, such as hepatic clearances, partition coefficients, and intestinal absorption. Our work allowed to estimate the prenatal exposure to permethrin in rats, especially in the fetal brain, and to quantitatively estimate the placental transfer. These transfers could be extrapolated to humans and be incorporated in a human pPBPK model to estimate the fetal exposure to permethrin from biomonitoring data.
Keywords: PBPK model; brain; fetus; permethrin; pesticides; pregnancy; pyrethroids; rat.
Copyright © 2021 Personne, Brochot, Marcelo, Corona, Desmots, Robidel, Lecomte, Bach and Zeman.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
PBPK modeling to support risk assessment of pyrethroid exposure in French pregnant women.Environ Res. 2024 Jun 15;251(Pt 1):118606. doi: 10.1016/j.envres.2024.118606. Epub 2024 Mar 8. Environ Res. 2024. PMID: 38460660
-
Determination of maternal and foetal distribution of cis- and trans-permethrin isomers and their metabolites in pregnant rats by liquid chromatography tandem mass spectrometry (LC-MS/MS).Anal Bioanal Chem. 2019 Dec;411(30):8043-8052. doi: 10.1007/s00216-019-02157-7. Epub 2019 Nov 20. Anal Bioanal Chem. 2019. PMID: 31748895
-
PBPK modeling of the cis- and trans-permethrin isomers and their major urinary metabolites in rats.Toxicol Appl Pharmacol. 2016 Mar 1;294:65-77. doi: 10.1016/j.taap.2016.01.011. Epub 2016 Jan 20. Toxicol Appl Pharmacol. 2016. PMID: 26802525
-
Parameters for pyrethroid insecticide QSAR and PBPK/PD models for human risk assessment.Rev Environ Contam Toxicol. 2012;219:1-114. doi: 10.1007/978-1-4614-3281-4_1. Rev Environ Contam Toxicol. 2012. PMID: 22610175 Review.
-
Methodological Approaches to Evaluate Fetal Drug Exposure.Curr Pharm Des. 2019;25(5):496-504. doi: 10.2174/1381612825666190319102812. Curr Pharm Des. 2019. PMID: 30892158 Review.
Cited by
-
Pesticide exposure, birth size, and gestational age in the ISA birth cohort, Costa Rica.Environ Epidemiol. 2024 Feb 14;8(2):e290. doi: 10.1097/EE9.0000000000000290. eCollection 2024 Apr. Environ Epidemiol. 2024. PMID: 38617432 Free PMC article.
-
Exposure of pregnant women and their children to pyrethroid insecticides in Rio de Janeiro, Brazil.Front Public Health. 2023 Dec 14;11:1274724. doi: 10.3389/fpubh.2023.1274724. eCollection 2023. Front Public Health. 2023. PMID: 38162602 Free PMC article.
-
Considering developmental neurotoxicity in vitro data for human health risk assessment using physiologically-based kinetic modeling: deltamethrin case study.Toxicol Sci. 2023 Mar 20;192(1):59-70. doi: 10.1093/toxsci/kfad007. Toxicol Sci. 2023. PMID: 36637193 Free PMC article.
-
Development of a Physiologically Based Pharmacokinetic (PBPK) Model for F-53B in Pregnant Mice and Its Extrapolation to Humans.Environ Sci Technol. 2024 Oct 22;58(42):18928-18939. doi: 10.1021/acs.est.4c05405. Epub 2024 Oct 12. Environ Sci Technol. 2024. PMID: 39394996 Free PMC article.
References
LinkOut - more resources
Full Text Sources

