Mechanistic Coupling of a Novel in silico Cotyledon Perfusion Model and a Physiologically Based Pharmacokinetic Model to Predict Fetal Acetaminophen Pharmacokinetics at Delivery
- PMID: 34631628
- PMCID: PMC8496351
- DOI: 10.3389/fped.2021.733520
Mechanistic Coupling of a Novel in silico Cotyledon Perfusion Model and a Physiologically Based Pharmacokinetic Model to Predict Fetal Acetaminophen Pharmacokinetics at Delivery
Abstract
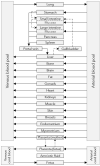
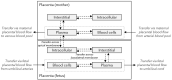
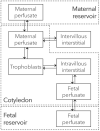
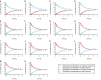
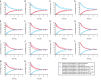
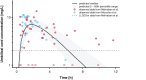
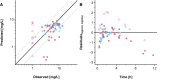
Little is known about placental drug transfer and fetal pharmacokinetics despite increasing drug use in pregnant women. While physiologically based pharmacokinetic (PBPK) models can help in some cases to shed light on this knowledge gap, adequate parameterization of placental drug transfer remains challenging. A novel in silico model with seven compartments representing the ex vivo cotyledon perfusion assay was developed and used to describe placental transfer and fetal pharmacokinetics of acetaminophen. Unknown parameters were optimized using observed data. Thereafter, values of relevant model parameters were copied to a maternal-fetal PBPK model and acetaminophen pharmacokinetics were predicted at delivery after oral administration of 1,000 mg. Predictions in the umbilical vein were evaluated with data from two clinical studies. Simulations from the in silico cotyledon perfusion model indicated that acetaminophen accumulates in the trophoblasts; simulated steady state concentrations in the trophoblasts were 4.31-fold higher than those in the perfusate. The whole-body PBPK model predicted umbilical vein concentrations with a mean prediction error of 24.7%. Of the 62 concentration values reported in the clinical studies, 50 values (81%) were predicted within a 2-fold error range. In conclusion, this study presents a novel in silico cotyledon perfusion model that is structurally congruent with the placenta implemented in our maternal-fetal PBPK model. This allows transferring parameters from the former model into our PBPK model for mechanistically exploring whole-body pharmacokinetics and concentration-effect relationships in the placental tissue. Further studies should investigate acetaminophen accumulation and metabolism in the placenta as the former might potentially affect placental prostaglandin synthesis and subsequent fetal exposure.
Keywords: acetaminophen; ex vivo cotyledon perfusion; maternal-fetal; physiologically-based pharmacokinetics; placental transfer; pregnancy.
Copyright © 2021 Mian, Nolan, van den Anker, van Calsteren, Allegaert, Lakhi and Dallmann.
Conflict of interest statement
AD is an employee of Bayer AG and uses Open Systems Pharmacology software, tools, and models in his professional role. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Integration of Placental Transfer in a Fetal-Maternal Physiologically Based Pharmacokinetic Model to Characterize Acetaminophen Exposure and Metabolic Clearance in the Fetus.Clin Pharmacokinet. 2020 Jul;59(7):911-925. doi: 10.1007/s40262-020-00861-7. Clin Pharmacokinet. 2020. PMID: 32052378 Free PMC article.
-
Predicting fetal exposure of crizotinib during pregnancy: Combining human ex vivo placenta perfusion data with physiologically-based pharmacokinetic modeling.Toxicol In Vitro. 2022 Dec;85:105471. doi: 10.1016/j.tiv.2022.105471. Epub 2022 Sep 10. Toxicol In Vitro. 2022. PMID: 36096459
-
Mechanistic Modeling of Placental Drug Transfer in Humans: How Do Differences in Maternal/Fetal Fraction of Unbound Drug and Placental Influx/Efflux Transfer Rates Affect Fetal Pharmacokinetics?Front Pediatr. 2021 Oct 18;9:723006. doi: 10.3389/fped.2021.723006. eCollection 2021. Front Pediatr. 2021. PMID: 34733804 Free PMC article.
-
Methodological Approaches to Evaluate Fetal Drug Exposure.Curr Pharm Des. 2019;25(5):496-504. doi: 10.2174/1381612825666190319102812. Curr Pharm Des. 2019. PMID: 30892158 Review.
-
Drug Transporters Expressed in the Human Placenta and Models for Studying Maternal-Fetal Drug Transfer.J Clin Pharmacol. 2019 Sep;59 Suppl 1(Suppl 1):S70-S81. doi: 10.1002/jcph.1491. J Clin Pharmacol. 2019. PMID: 31502693 Free PMC article. Review.
Cited by
-
Recent advances and current challenges of new approach methodologies in developmental and adult neurotoxicity testing.Arch Toxicol. 2024 May;98(5):1271-1295. doi: 10.1007/s00204-024-03703-8. Epub 2024 Mar 13. Arch Toxicol. 2024. PMID: 38480536 Free PMC article. Review.
-
Considering developmental neurotoxicity in vitro data for human health risk assessment using physiologically-based kinetic modeling: deltamethrin case study.Toxicol Sci. 2023 Mar 20;192(1):59-70. doi: 10.1093/toxsci/kfad007. Toxicol Sci. 2023. PMID: 36637193 Free PMC article.
-
Applications, Challenges, and Outlook for PBPK Modeling and Simulation: A Regulatory, Industrial and Academic Perspective.Pharm Res. 2022 Aug;39(8):1701-1731. doi: 10.1007/s11095-022-03274-2. Epub 2022 May 13. Pharm Res. 2022. PMID: 35552967 Review.
-
In Silico Modeling for Ex Vivo Placental Transfer of Morphine.J Clin Pharmacol. 2022 Sep;62 Suppl 1(Suppl 1):140-146. doi: 10.1002/jcph.2105. J Clin Pharmacol. 2022. PMID: 36106779 Free PMC article.
-
Physiologically Based Pharmacokinetic Modeling in Pregnant Women Suggests Minor Decrease in Maternal Exposure to Olanzapine.Front Pharmacol. 2022 Jan 19;12:793346. doi: 10.3389/fphar.2021.793346. eCollection 2021. Front Pharmacol. 2022. PMID: 35126130 Free PMC article.
References
-
- Codaccioni M, Bois F, Brochot C. Placental transfer of xenobiotics in pregnancy physiologically-based pharmacokinetic models: structure and data. Computational Toxicol. (2019) 12:100111. 10.1016/j.comtox.2019.100111 - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

