Laminin-1 Peptides Conjugated to Fibrin Hydrogels Promote Salivary Gland Regeneration in Irradiated Mouse Submandibular Glands
- PMID: 34631679
- PMCID: PMC8498954
- DOI: 10.3389/fbioe.2021.729180
Laminin-1 Peptides Conjugated to Fibrin Hydrogels Promote Salivary Gland Regeneration in Irradiated Mouse Submandibular Glands
Abstract
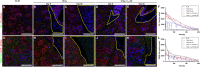
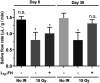
Previous studies demonstrated that salivary gland morphogenesis and differentiation are enhanced by modification of fibrin hydrogels chemically conjugated to Laminin-1 peptides. Specifically, Laminin-1 peptides (A99: CGGALRGDN-amide and YIGSR: CGGADPGYIGSRGAA-amide) chemically conjugated to fibrin promoted formation of newly organized salivary epithelium both in vitro (e.g., using organoids) and in vivo (e.g., in a wounded mouse model). While these studies were successful, the model's usefulness for inducing regenerative patterns after radiation therapy remains unknown. Therefore, the goal of the current study was to determine whether transdermal injection with the Laminin-1 peptides A99 and YIGSR chemically conjugated to fibrin hydrogels promotes tissue regeneration in irradiated salivary glands. Results indicate that A99 and YIGSR chemically conjugated to fibrin hydrogels promote formation of functional salivary tissue when transdermally injected to irradiated salivary glands. In contrast, when left untreated, irradiated salivary glands display a loss in structure and functionality. Together, these studies indicate that fibrin hydrogel-based implantable scaffolds containing Laminin-1 peptides promote secretory function of irradiated salivary glands.
Keywords: biomaterial; hydrogel; irradiated salivary glands; regeneration; saliva; tissue engineering.
Copyright © 2021 Nam, dos Santos, Maslow, Trump, Lei, Andreadis and Baker.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Fibrin hydrogels fortified with FGF-7/10 and laminin-1 peptides promote regeneration of irradiated salivary glands.Acta Biomater. 2023 Dec;172:147-158. doi: 10.1016/j.actbio.2023.10.013. Epub 2023 Oct 14. Acta Biomater. 2023. PMID: 37844750 Free PMC article.
-
L1 Peptide-Conjugated Fibrin Hydrogels Promote Salivary Gland Regeneration.J Dent Res. 2017 Jul;96(7):798-806. doi: 10.1177/0022034517695496. Epub 2017 Feb 16. J Dent Res. 2017. PMID: 28208029 Free PMC article.
-
Synergistic effects of laminin-1 peptides, VEGF and FGF9 on salivary gland regeneration.Acta Biomater. 2019 Jun;91:186-194. doi: 10.1016/j.actbio.2019.04.049. Epub 2019 Apr 25. Acta Biomater. 2019. PMID: 31028910 Free PMC article.
-
Molecular cues for development and regeneration of salivary glands.Histol Histopathol. 2014 Mar;29(3):305-12. doi: 10.14670/HH-29.305. Epub 2013 Nov 5. Histol Histopathol. 2014. PMID: 24189993 Free PMC article. Review.
-
Trends in Salivary Gland Tissue Engineering: From Stem Cells to Secretome and Organoid Bioprinting.Tissue Eng Part B Rev. 2021 Apr;27(2):155-165. doi: 10.1089/ten.TEB.2020.0149. Epub 2020 Aug 26. Tissue Eng Part B Rev. 2021. PMID: 32723016 Review.
Cited by
-
Salivary Gland Bioengineering.Bioengineering (Basel). 2023 Dec 26;11(1):28. doi: 10.3390/bioengineering11010028. Bioengineering (Basel). 2023. PMID: 38247905 Free PMC article. Review.
-
Polymer-Based Scaffolds as an Implantable Material in Regenerative Dentistry: A Review.J Funct Biomater. 2025 Feb 24;16(3):80. doi: 10.3390/jfb16030080. J Funct Biomater. 2025. PMID: 40137359 Free PMC article. Review.
-
Intestinal organoid modeling: bridging the gap from experimental model to clinical translation.Front Oncol. 2024 Mar 1;14:1334631. doi: 10.3389/fonc.2024.1334631. eCollection 2024. Front Oncol. 2024. PMID: 38496762 Free PMC article. Review.
-
Fibrin hydrogels fortified with FGF-7/10 and laminin-1 peptides promote regeneration of irradiated salivary glands.Acta Biomater. 2023 Dec;172:147-158. doi: 10.1016/j.actbio.2023.10.013. Epub 2023 Oct 14. Acta Biomater. 2023. PMID: 37844750 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources

