Single-Molecule Imaging Reveals Rapid Estradiol Action on the Surface Movement of AMPA Receptors in Live Neurons
- PMID: 34631701
- PMCID: PMC8495425
- DOI: 10.3389/fcell.2021.708715
Single-Molecule Imaging Reveals Rapid Estradiol Action on the Surface Movement of AMPA Receptors in Live Neurons
Abstract
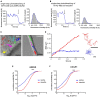
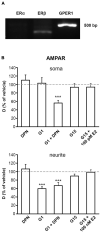
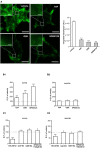
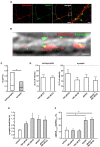
Gonadal steroid 17β-estradiol (E2) exerts rapid, non-genomic effects on neurons and strictly regulates learning and memory through altering glutamatergic neurotransmission and synaptic plasticity. However, its non-genomic effects on AMPARs are not well understood. Here, we analyzed the rapid effect of E2 on AMPARs using single-molecule tracking and super-resolution imaging techniques. We found that E2 rapidly decreased the surface movement of AMPAR via membrane G protein-coupled estrogen receptor 1 (GPER1) in neurites in a dose-dependent manner. The cortical actin network played a pivotal role in the GPER1 mediated effects of E2 on the surface mobility of AMPAR. E2 also decreased the surface movement of AMPAR both in synaptic and extrasynaptic regions on neurites and increased the synaptic dwell time of AMPARs. Our results provide evidence for understanding E2 action on neuronal plasticity and glutamatergic neurotransmission at the molecular level.
Keywords: 17β-estradiol; AMPAR; diffusion; single-molecule tracking; synapse.
Copyright © 2021 Godó, Barabás, Lengyel, Ernszt, Kovács, Kecskés, Varga, Jánosi, Makkai, Kovács, Orsolits, Fujiwara, Kusumi and Ábrahám.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Bálint F., Liposits Z., Farkas I. (2016). Estrogen receptor beta and 2-arachidonoylglycerol mediate the suppressive effects of estradiol on frequency of postsynaptic currents in gonadotropin-releasing hormone neurons of metestrous mice: an acute slice electrophysiological study. Front. Cell Neurosci. 10:77. 10.3389/fncel.2016.00077 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

