Amlexanox and Forskolin Prevents Isoproterenol-Induced Cardiomyopathy by Subduing Cardiomyocyte Hypertrophy and Maladaptive Inflammatory Responses
- PMID: 34631707
- PMCID: PMC8497899
- DOI: 10.3389/fcell.2021.719351
Amlexanox and Forskolin Prevents Isoproterenol-Induced Cardiomyopathy by Subduing Cardiomyocyte Hypertrophy and Maladaptive Inflammatory Responses
Abstract
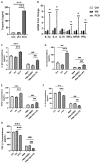
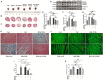
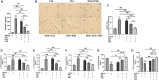
Chronic catecholamine stress (CCS) induces the occurrence of cardiomyopathy-pathological cardiac hypertrophy (PCH), which is characterized by left ventricular systolic dysfunction (LVSD). Recently, mounting evidence has implicated myocardial inflammation in the exacerbation of pathological cardiac remodeling. However, there are currently no well-defined treatment interventions or regimes targeted at both the attenuation of maladaptive myocardial hypertrophy and inflammation during CCS to prevent PCH. G protein-coupled receptor kinase 5 (GRK5) and adenylyl cyclases (ACs)-cAMP mediates both cardiac and inflammatory responses. Also, GRK5 and ACs are implicated in stress-induced LVSD. Herein, we aimed at preventing PCH during CCS via modulating adaptive cardiac and inflammatory responses by inhibiting GRK5 and/or stimulating ACs. Isoproterenol-induced cardiomyopathy (ICM) was modeled using 0.5 mg/100 g/day isoproterenol injections for 40 days. Alterations in cardiac and inflammatory responses were assessed from the myocardia. Similarities in the immunogenicity of cardiac troponin I (cTnI) and lipopolysaccharide under CCS were assessed, and Amlexanox (35 μM/ml) and/or Forskolin (10 μM/ml) were then employed in vitro to modulate adaptive inflammatory responses by inhibiting GRK5 or activating ACs-cAMP, respectively. Subsequently, Amlexanox (2.5 mg/100 g/day) and/or Forskolin (0.5 mg/100 g/day) were then translated into in vivo during CCS to modulate adaptive cardiac and inflammatory responses. The effects of Amlexanox and Forskolin on regulating myocardial systolic functions and inflammatory responses during CCS were ascertained afterward. PCH mice had excessive myocardial hypertrophy, fibrosis, and aggravated LVSD, which were accompanied by massive CD68+ inflammatory cell infiltrations. In vitro, Forskolin-AC/cAMP was effective than Amlexanox-GRK5 at downregulating proinflammatory responses during stress; nonetheless, Amlexanox and Forskolin combination demonstrated the most efficacy in modulating adaptive inflammatory responses. Individually, the translated Amlexanox and Forskolin treatment interventions were ineffective at subduing the pathological remodeling and sustaining cardiac function during CCS. However, their combination was potent at preventing LVSD during CCS by attenuating maladaptive myocardial hypertrophy, fibrosis, and inflammatory responses. The treatment intervention attained its potency mainly via Forskolin-ACs/cAMP-mediated modulation of cardiac and inflammatory responses, coupled with Amlexanox inhibition of GRK5 mediated maladaptive cascades. Taken together, our findings highlight the Amlexanox and Forskolin combination as a potential therapeutic intervention for preventing the occurrence of pathological cardiac hypertrophy during chronic stress.
Keywords: GRK5; adenylyl cyclase; amlexanox; cAMP; chronic catecholamine stress; forskolin; inflammation; isoproterenol-induced cardiomyopathy.
Copyright © 2021 Adzika, Hou, Adekunle, Rizvi, Adzraku, Li, Deng, Mprah, Ndzie Noah, Adu-Amankwaah, Machuki, Shang, Ma, Koda, Ma and Sun.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Isoproterenol-Induced Cardiomyopathy Recovery Intervention: Amlexanox and Forskolin Enhances the Resolution of Catecholamine Stress-Induced Maladaptive Myocardial Remodeling.Front Cardiovasc Med. 2021 Nov 25;8:719805. doi: 10.3389/fcvm.2021.719805. eCollection 2021. Front Cardiovasc Med. 2021. PMID: 34901202 Free PMC article.
-
Estrogen Attenuates Chronic Stress-Induced Cardiomyopathy by Adaptively Regulating Macrophage Polarizations via β2-Adrenergic Receptor Modulation.Front Cell Dev Biol. 2021 Sep 28;9:737003. doi: 10.3389/fcell.2021.737003. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34650984 Free PMC article.
-
KR-39038, a Novel GRK5 Inhibitor, Attenuates Cardiac Hypertrophy and Improves Cardiac Function in Heart Failure.Biomol Ther (Seoul). 2020 Sep 1;28(5):482-489. doi: 10.4062/biomolther.2020.129. Biomol Ther (Seoul). 2020. PMID: 32856617 Free PMC article.
-
GRK2 and GRK5 as therapeutic targets and their role in maladaptive and pathological cardiac hypertrophy.Expert Opin Ther Targets. 2019 Mar;23(3):201-214. doi: 10.1080/14728222.2019.1575363. Epub 2019 Feb 13. Expert Opin Ther Targets. 2019. PMID: 30701991 Review.
-
Pathological cardiac hypertrophy: the synergy of adenylyl cyclases inhibition in cardiac and immune cells during chronic catecholamine stress.J Mol Med (Berl). 2019 Jul;97(7):897-907. doi: 10.1007/s00109-019-01790-0. Epub 2019 May 6. J Mol Med (Berl). 2019. PMID: 31062036 Review.
Cited by
-
Integrated MicroRNA-mRNA Sequencing Analysis Identifies Regulators and Networks Involved in Feline Hypertrophic Cardiomyopathy.Int J Mol Sci. 2025 Jul 15;26(14):6764. doi: 10.3390/ijms26146764. Int J Mol Sci. 2025. PMID: 40725010 Free PMC article.
-
Estrogen downregulates CD73/adenosine axis hyperactivity via adaptive modulation PI3K/Akt signaling to prevent myocarditis and arrhythmias during chronic catecholamines stress.Cell Commun Signal. 2023 Feb 23;21(1):41. doi: 10.1186/s12964-023-01052-0. Cell Commun Signal. 2023. PMID: 36823590 Free PMC article.
-
ADAM17, A Key Player of Cardiac Inflammation and Fibrosis in Heart Failure Development During Chronic Catecholamine Stress.Front Cell Dev Biol. 2021 Dec 13;9:732952. doi: 10.3389/fcell.2021.732952. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34966735 Free PMC article. Review.
-
Isoproterenol mechanisms in inducing myocardial fibrosis and its application as an experimental model for the evaluation of therapeutic potential of phytochemicals and pharmaceuticals.Animal Model Exp Med. 2025 Jan;8(1):67-91. doi: 10.1002/ame2.12496. Epub 2024 Dec 17. Animal Model Exp Med. 2025. PMID: 39690876 Free PMC article. Review.
-
Amlexanox reduces new-onset atrial fibrillation risk in sepsis by downregulating S100A12: a Mendelian randomization study.Front Cardiovasc Med. 2024 Oct 9;11:1401314. doi: 10.3389/fcvm.2024.1401314. eCollection 2024. Front Cardiovasc Med. 2024. PMID: 39444551 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials

