Desmosomes as Signaling Hubs in the Regulation of Cell Behavior
- PMID: 34631720
- PMCID: PMC8495202
- DOI: 10.3389/fcell.2021.745670
Desmosomes as Signaling Hubs in the Regulation of Cell Behavior
Abstract
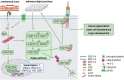
Desmosomes are intercellular junctions, which preserve tissue integrity during homeostatic and stress conditions. These functions rely on their unique structural properties, which enable them to respond to context-dependent signals and transmit them to change cell behavior. Desmosome composition and size vary depending on tissue specific expression and differentiation state. Their constituent proteins are highly regulated by posttranslational modifications that control their function in the desmosome itself and in addition regulate a multitude of desmosome-independent functions. This review will summarize our current knowledge how signaling pathways that control epithelial shape, polarity and function regulate desmosomes and how desmosomal proteins transduce these signals to modulate cell behavior.
Keywords: EGFR; Hippo signaling; IGF1R; barrier function; desmosomes; differentiation; inflammation; proliferation.
Copyright © 2021 Müller, Hatzfeld and Keil.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

