Prompt Reduction in CRP, IL-6, IFN-γ, IP-10, and MCP-1 and a Relatively Low Basal Ratio of Ferritin/CRP Is Possibly Associated With the Efficacy of Tocilizumab Monotherapy in Severely to Critically Ill Patients With COVID-19
- PMID: 34631752
- PMCID: PMC8494777
- DOI: 10.3389/fmed.2021.734838
Prompt Reduction in CRP, IL-6, IFN-γ, IP-10, and MCP-1 and a Relatively Low Basal Ratio of Ferritin/CRP Is Possibly Associated With the Efficacy of Tocilizumab Monotherapy in Severely to Critically Ill Patients With COVID-19
Abstract
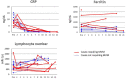
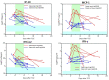
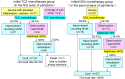
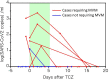
Background and Aim: Tocilizumab, a humanized anti-IL-6 receptor antibody, has been used to treat severely to critically ill patients with COVID-19. A living systematic review with meta-analysis of recent RCTs indicates that the combination therapy of corticosteroids and tocilizumab produce better outcomes, while previous observational studies suggest that tocilizumab monotherapy is beneficial for substantial numbers of patients. However, what patients could respond to tocilizumab monotherapy remained unknown. Methods: In this retrospective study we evaluated the effects of tocilizumab monotherapy on the clinical characteristics, serum biomediator levels, viral elimination, and specific IgG antibody induction in 13 severely to critically ill patients and compared with those of dexamethasone monotherapy and dexamethasone plus tocilizumab. Results: A single tocilizumab administration led to a rapid improvement in clinical characteristics, inflammatory findings, and oxygen supply in 7 of 11 patients with severe COVID-19, and could recover from mechanical ventilation management (MVM) in 2 patients with critically ill COVID-19. Four patients exhibited rapidly worsening even after tocilizumab administration and required MVM and additional methylprednisolone treatment. Tocilizumab did not delay viral elimination or inhibit IgG production specific for the virus, whereas dexamethasone inhibited IgG induction. A multiplex cytokine array system revealed a significant increase in the serum expression of 54 out of 80 biomediators in patients with COVID-19 compared with that in healthy controls. Compared with those who promptly recovered in response to tocilizumab, patients requiring MVM showed a significantly higher ratio of basal level of ferritin/CRP and a persistent increase in the levels of CRP and specific cytokines and chemokines including IL-6, IFN-γ, IP-10, and MCP-1. The basal high ratio of ferritin/CRP was also associated with clinical deterioration even in patients treated with dexamethasone and tocilizumab. Conclusion: Tocilizumab as monotherapy has substantial beneficial effects in some patients with severe COVID-19, who showed a relatively low level of the ratio of ferritin/CRP and prompt reduction in CRP, IL-6, IFN-γ, IP-10, and MCP-1. The high ratio of ferritin/CRP is associated with rapid worsening of pneumonia. Further evaluation is warranted to clarify whether tocilizumab monotherapy or its combination with corticosteroid is preferred for severely to critically ill patients with COVID-19.
Keywords: COVID-19; IL-6; cytokine storm; dexamethasone; tocilizumab.
Copyright © 2021 Hashimoto, Yoshizaki, Uno, Kitajima, Arai, Tamura, Morishita, Matsuoka, Han, Minamoto, Hirashima, Yamada, Kashiwa, Kameda, Yamaguchi, Tsuchihashi, Iwahashi, Nakayama, Shioda, Nagai and Tanaka.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- World Health Organization . Coronavirus Disease 2019 (COVID-19) Situation Report-51. Geneva: World Health Organization; (2020). Available online at: http://www.who.int/docs/default-source/coronaviruse/situation-reports/20... (accessed March 11, 2020).
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

