Amnion-Derived Mesenchymal Stromal/Stem Cell Paracrine Signals Potentiate Human Liver Organoid Differentiation: Translational Implications for Liver Regeneration
- PMID: 34631757
- PMCID: PMC8494784
- DOI: 10.3389/fmed.2021.746298
Amnion-Derived Mesenchymal Stromal/Stem Cell Paracrine Signals Potentiate Human Liver Organoid Differentiation: Translational Implications for Liver Regeneration
Abstract
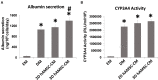
The prevalence of end-stage liver diseases has reached very high levels globally. The election treatment for affected patients is orthotopic liver transplantation, which is a very complex procedure, and due to the limited number of suitable organ donors, considerable research is being done on alternative therapeutic options. For instance, the use of cell therapy, such as the transplantation of hepatocytes to promote liver repair/regeneration, has been explored, but standardized protocols to produce suitable human hepatocytes are still limited. On the other hand, liver progenitor and multipotent stem cells offer potential cell sources that could be used clinically. Different studies have reported regarding the therapeutic effects of transplanted mesenchymal stromal/stem cells (MSCs) on end-stage liver diseases. Moreover, it has been shown that delivery of MSC-derived conditioned medium (MSC-CM) can reduce cell death and enhance liver proliferation in fulminant hepatic failure. Therefore, it is believed that MSC-CM contains many factors that probably support liver regeneration. In our work, we used an in vitro model of human liver organoids to study if the paracrine components secreted by human amnion-derived MSCs (hAMSCs) affected liver stem/progenitor cell differentiation. In particular, we differentiated liver organoids derived from bipotent EpCAM+ human liver cells and tested the effects of hAMSC secretome, derived from both two-dimensional (2D) and three-dimensional (3D) hAMSC cultures, on that model. Our analysis showed that conditioned medium (CM) produced by 3D hAMSCs was able to induce an over-expression of mature hepatocyte markers, such as ALB, NTCP, and CYP3A4, compared with both 2D hAMSC cultures and the conventional differentiation medium (DM). These data were confirmed by the over-production of ALB protein and over-activity of CYP3A4 observed in organoids grown in 3D hAMSC-CM. Liver repair dysfunction plays a role in the development of liver diseases, and effective repair likely requires the normal functioning of liver stem/progenitor cells. Herein, we showed that hAMSC-CM produced mainly by 3D cultures had the potential to increase hepatic stem/progenitor cell differentiation, demonstrating that soluble factors secreted by those cells are potentially responsible for the reaction. This work shows a potential approach to improve liver repair/regeneration also in a transplantation setting.
Keywords: 3D liver organoid culture; hepatic progenitor cell differentiation; hepatocyte culture; human amnion-derived mesenchymal stem cells; liver regeneration.
Copyright © 2021 Lo Nigro, Gallo, Bulati, Vitale, Paini, Pampalone, Galvagno, Conaldi and Miceli.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Human amniotic mesenchymal stem cells and their paracrine factors promote wound healing by inhibiting heat stress-induced skin cell apoptosis and enhancing their proliferation through activating PI3K/AKT signaling pathway.Stem Cell Res Ther. 2019 Aug 9;10(1):247. doi: 10.1186/s13287-019-1366-y. Stem Cell Res Ther. 2019. PMID: 31399039 Free PMC article.
-
Changes in the Transcriptome Profiles of Human Amnion-Derived Mesenchymal Stromal/Stem Cells Induced by Three-Dimensional Culture: A Potential Priming Strategy to Improve Their Properties.Int J Mol Sci. 2022 Jan 13;23(2):863. doi: 10.3390/ijms23020863. Int J Mol Sci. 2022. PMID: 35055049 Free PMC article.
-
Comparison of Immunosuppressive and Angiogenic Properties of Human Amnion-Derived Mesenchymal Stem Cells between 2D and 3D Culture Systems.Stem Cells Int. 2019 Feb 18;2019:7486279. doi: 10.1155/2019/7486279. eCollection 2019. Stem Cells Int. 2019. PMID: 30911299 Free PMC article.
-
Pluripotent-Stem-Cell-Derived Hepatic Cells: Hepatocytes and Organoids for Liver Therapy and Regeneration.Cells. 2020 Feb 12;9(2):420. doi: 10.3390/cells9020420. Cells. 2020. PMID: 32059501 Free PMC article. Review.
-
Hepatocyte transplantation and advancements in alternative cell sources for liver-based regenerative medicine.J Mol Med (Berl). 2018 Jun;96(6):469-481. doi: 10.1007/s00109-018-1638-5. Epub 2018 Apr 24. J Mol Med (Berl). 2018. PMID: 29691598 Free PMC article. Review.
Cited by
-
3D Culture and Interferon-γ Priming Modulates Characteristics of Mesenchymal Stromal/Stem Cells by Modifying the Expression of Both Intracellular and Exosomal microRNAs.Biology (Basel). 2023 Jul 28;12(8):1063. doi: 10.3390/biology12081063. Biology (Basel). 2023. PMID: 37626949 Free PMC article.
-
Proteomic analysis and functional validation reveal distinct therapeutic capabilities related to priming of mesenchymal stromal/stem cells with IFN-γ and hypoxia: potential implications for their clinical use.Front Cell Dev Biol. 2024 May 31;12:1385712. doi: 10.3389/fcell.2024.1385712. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 38882056 Free PMC article.
-
Human Amniotic MSC Response in LPS-Stimulated Ascites from Patients with Cirrhosis: FOXO1 Gene and Th17 Activation in Enhanced Antibacterial Activation.Int J Mol Sci. 2024 Feb 28;25(5):2801. doi: 10.3390/ijms25052801. Int J Mol Sci. 2024. PMID: 38474048 Free PMC article.
-
Use of priming strategies to advance the clinical application of mesenchymal stromal/stem cell-based therapy.World J Stem Cells. 2024 Jan 26;16(1):7-18. doi: 10.4252/wjsc.v16.i1.7. World J Stem Cells. 2024. PMID: 38292438 Free PMC article.
-
Liver Regeneration and Cell Transplantation for End-Stage Liver Disease.Biomolecules. 2021 Dec 20;11(12):1907. doi: 10.3390/biom11121907. Biomolecules. 2021. PMID: 34944550 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

