Adeno-Associated Virus-Mediated Gain-of-Function mPCSK9 Expression in the Mouse Induces Hypercholesterolemia, Monocytosis, Neutrophilia, and a Hypercoagulative State
- PMID: 34631822
- PMCID: PMC8492965
- DOI: 10.3389/fcvm.2021.718741
Adeno-Associated Virus-Mediated Gain-of-Function mPCSK9 Expression in the Mouse Induces Hypercholesterolemia, Monocytosis, Neutrophilia, and a Hypercoagulative State
Erratum in
-
Corrigendum: Adeno-Associated Virus-Mediated Gain-of-Function mPCSK9 Expression in the Mouse Induces Hypercholesterolemia, Monocytosis, Neutrophilia, and a Hypercoagulative State.Front Cardiovasc Med. 2022 Jan 12;8:820282. doi: 10.3389/fcvm.2021.820282. eCollection 2021. Front Cardiovasc Med. 2022. PMID: 35097032 Free PMC article.
Abstract
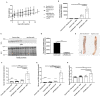
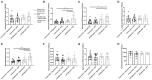
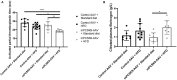
Hypercholesterolemia has previously been induced in the mouse by a single intravenous injection of adeno-associated virus (AAV)-based vector harboring gain-of-function pro-protein convertase subtilisin/kexin type 9. Despite the recent emergence of the PCSK9-AAV model, the profile of hematological and coagulation parameters associated with it has yet to be characterized. We injected 1.0 × 1011 viral particles of mPCSK9-AAV or control AAV into juvenile male C57BL/6N mice and fed them with either a Western-type high-fat diet (HFD) or standard diet over the course of 3 weeks. mPCSK9-AAV mice on HFD exhibited greater plasma PCSK9 concentration and lower low-density lipoprotein levels, concomitant with increased total cholesterol and non-high-density lipoprotein (non-HDL)-cholesterol concentrations, and lower HDL-cholesterol concentrations than control mice. Furthermore, mPCSK9-AAV-injected mice on HFD exhibited no signs of atherosclerosis at 3 weeks after the AAV injection. Hypercholesterolemia was associated with a thromboinflammatory phenotype, as neutrophil levels, monocyte levels, and neutrophil-to-lymphocyte ratios were higher and activated partial thromboplastin times (aPTTs) was lower in HFD-fed mPCSK9-AAV mice. Therefore, the mPCSK9-AAV is a suitable model of hypercholesterolemia to examine the role of thromboinflammatory processes in the pathogenesis of cardiovascular and cerebrovascular diseases.
Keywords: PCSK9; coagulation; hypercholesterolemia; monocytes; mouse; neutrophils.
Copyright © 2021 Louloudis, Ambrosini, Paneni, Camici, Benke and Klohs.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Hypercholesterolemia Induced by a PCSK9 Gain-of-Function Mutation Augments Angiotensin II-Induced Abdominal Aortic Aneurysms in C57BL/6 Mice-Brief Report.Arterioscler Thromb Vasc Biol. 2016 Sep;36(9):1753-7. doi: 10.1161/ATVBAHA.116.307613. Epub 2016 Jul 28. Arterioscler Thromb Vasc Biol. 2016. PMID: 27470509 Free PMC article.
-
Induction of sustained hypercholesterolemia by single adeno-associated virus-mediated gene transfer of mutant hPCSK9.Arterioscler Thromb Vasc Biol. 2015 Jan;35(1):50-9. doi: 10.1161/ATVBAHA.114.303617. Epub 2014 Oct 23. Arterioscler Thromb Vasc Biol. 2015. PMID: 25341796
-
Corrigendum: Adeno-Associated Virus-Mediated Gain-of-Function mPCSK9 Expression in the Mouse Induces Hypercholesterolemia, Monocytosis, Neutrophilia, and a Hypercoagulative State.Front Cardiovasc Med. 2022 Jan 12;8:820282. doi: 10.3389/fcvm.2021.820282. eCollection 2021. Front Cardiovasc Med. 2022. PMID: 35097032 Free PMC article.
-
Targeting the proprotein convertase subtilisin/kexin type 9 for the treatment of dyslipidemia and atherosclerosis.J Am Coll Cardiol. 2013 Oct 15;62(16):1401-8. doi: 10.1016/j.jacc.2013.07.056. Epub 2013 Aug 21. J Am Coll Cardiol. 2013. PMID: 23973703 Review.
-
Proprotein convertase subtilisin/kexin type 9 (PCSK9) in lipid metabolism, atherosclerosis and ischemic stroke.Int J Neurosci. 2016 Aug;126(8):675-80. doi: 10.3109/00207454.2015.1057636. Epub 2015 Jul 14. Int J Neurosci. 2016. PMID: 26040332 Review.
Cited by
-
Rosa26-LSL-dCas9-VPR: a versatile mouse model for tissue specific and simultaneous activation of multiple genes for drug discovery.Sci Rep. 2022 Nov 10;12(1):19268. doi: 10.1038/s41598-022-23127-7. Sci Rep. 2022. PMID: 36357523 Free PMC article.
-
Novel Insight Into Long-Term Risk of Major Adverse Cardiovascular and Cerebrovascular Events Following Lower Extremity Arteriosclerosis Obliterans.Front Cardiovasc Med. 2022 Apr 4;9:853583. doi: 10.3389/fcvm.2022.853583. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 35445093 Free PMC article.
-
Disturbed Flow Induces Reprogramming of Endothelial Cells to Immune-like and Foam Cells under Hypercholesterolemia during Atherogenesis.Res Sq [Preprint]. 2025 Mar 7:rs.3.rs-4397799. doi: 10.21203/rs.3.rs-4397799/v1. Res Sq. 2025. PMID: 40092444 Free PMC article. Preprint.
-
The impact of a humanized bile acid composition on atherosclerosis development in hypercholesterolaemic Cyp2c70 knockout mice.Sci Rep. 2025 Jan 15;15(1):2100. doi: 10.1038/s41598-025-86183-9. Sci Rep. 2025. PMID: 39815082 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

