Proteome Modulation in Peripheral Blood Mononuclear Cells of Peste des Petits Ruminants Vaccinated Goats and Sheep
- PMID: 34631844
- PMCID: PMC8493254
- DOI: 10.3389/fvets.2021.670968
Proteome Modulation in Peripheral Blood Mononuclear Cells of Peste des Petits Ruminants Vaccinated Goats and Sheep
Abstract
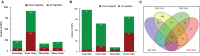
In the present study, healthy goats and sheep (n = 5) that were confirmed negative for peste des petits ruminants virus (PPRV) antibodies by monoclonal antibody-based competitive ELISA and by serum neutralization test and for PPRV antigen by s-ELISA were vaccinated with Sungri/96. A quantitative study was carried out to compare the proteome of peripheral blood mononuclear cells (PBMCs) of vaccinated goat and sheep [5 days post-vaccination (dpv) and 14 dpv] vs. unvaccinated (0 day) to divulge the alteration in protein expression following vaccination. A total of 232 and 915 proteins were differentially expressed at 5 and 14 dpv, respectively, in goats. Similarly, 167 and 207 proteins were differentially expressed at 5 and 14 dpv, respectively, in sheep. Network generated by Ingenuity Pathway Analysis was "infectious diseases, antimicrobial response, and inflammatory response," which includes the highest number of focus molecules. The bio functions, cell-mediated immune response, and humoral immune response were highly enriched in goats at 5 dpv and at 14 dpv. At the molecular level, the immune response produced by the PPRV vaccine virus in goats is effectively coordinated and stronger than that in sheep, though the vaccine provides protection from virulent virus challenge in both. The altered expression of certain PBMC proteins especially ISG15 and IRF7 induces marked changes in cellular signaling pathways to coordinate host immune responses.
Keywords: PPRV; Sungri/96; immunity; proteome; vaccine.
Copyright © 2021 Wani, Sahu, Khan, Praharaj, Saxena, Rajak, Muthuchelvan, Sahoo, Mishra, Singh, Mishra and Gandham.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Systems Biology behind Immunoprotection of Both Sheep and Goats after Sungri/96 PPRV Vaccination.mSystems. 2021 Mar 30;6(2):e00820-20. doi: 10.1128/mSystems.00820-20. mSystems. 2021. PMID: 33785572 Free PMC article.
-
Expression kinetics of ISG15, IRF3, IFNγ, IL10, IL2 and IL4 genes vis-a-vis virus shedding, tissue tropism and antibody dynamics in PPRV vaccinated, challenged, infected sheep and goats.Microb Pathog. 2018 Apr;117:206-218. doi: 10.1016/j.micpath.2018.02.027. Epub 2018 Feb 21. Microb Pathog. 2018. PMID: 29476787
-
A goat poxvirus-vectored peste-des-petits-ruminants vaccine induces long-lasting neutralization antibody to high levels in goats and sheep.Vaccine. 2010 Jul 5;28(30):4742-50. doi: 10.1016/j.vaccine.2010.04.102. Epub 2010 May 14. Vaccine. 2010. PMID: 20471441
-
Comparative and temporal transcriptome analysis of peste des petits ruminants virus infected goat peripheral blood mononuclear cells.Virus Res. 2017 Feb 2;229:28-40. doi: 10.1016/j.virusres.2016.12.014. Epub 2016 Dec 23. Virus Res. 2017. PMID: 28017736
-
Comparative efficacy of peste des petits ruminants (PPR) vaccines.Biologicals. 2010 Jul;38(4):479-85. doi: 10.1016/j.biologicals.2010.02.003. Epub 2010 Mar 2. Biologicals. 2010. PMID: 20199873 Review.
Cited by
-
Peste des petits ruminants virus virulence is associated with an early inflammatory profile in the tonsils and cell cycle arrest in lymphoid tissue.Microbiol Spectr. 2025 Apr;13(4):e0312424. doi: 10.1128/spectrum.03124-24. Epub 2025 Feb 24. Microbiol Spectr. 2025. PMID: 39992151 Free PMC article.
-
Comparative proteomic profiling of the ovine and human PBMC inflammatory response.Sci Rep. 2024 Jun 28;14(1):14939. doi: 10.1038/s41598-024-66059-0. Sci Rep. 2024. PMID: 38942936 Free PMC article.
References
-
- Shaila MS, Purushothaman V, Bhavasar D, Venugopal K, Venkatesan RA. Peste des petits ruminants of sheep in India. Vet Rec. (1989) 125:602. - PubMed
-
- Singh RK, Balamurugan V, Bhanuprakash V, Sen A, Saravanan P, Pal Yadav M. Possible control and eradication of peste des petits ruminants from India: technical aspects. Vet Ital. (2009) 45:449–62. - PubMed
-
- Diallo A, Taylor WP, Lefevre PC, Provost A. Attenuation of a strain of rinderpest virus: potential homologous live vaccine. Revue d'elevage et de medecine veterinaire des pays tropicaux. Rev Elev Med Vet Pays Trop. (1989) 42:311–9. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

