Adalimumab in patients with vision-threatening uveitis: real-world clinical experience
- PMID: 34632076
- PMCID: PMC8477319
- DOI: 10.1136/bmjophth-2021-000819
Adalimumab in patients with vision-threatening uveitis: real-world clinical experience
Abstract
Objectives: Biologics are rapidly emerging as an effective vision saving addition to systemic uveitis therapy. The aim of this multicentre retrospective study is to review the outcomes of a large group of patients treated with adalimumab.
Methods: A retrospective chart review of patients with refractory non-infectious, active uveitis treated with adalimumab was conducted. The main outcome measures were ability to reduce prednisolone dose, ability to control uveitis, final visual acuity and time to treatment failure.
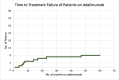
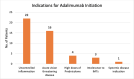
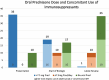
Results: Forty-six patients with uveitis, treated with adalimumab were included in the study. The most common anatomical uveitis phenotype was panuveitis (n=17, 37.0%). The most common diagnosis was idiopathic uveitis (n=19, 41.3%). At their latest review (mean: 4.46 years; median 4.40 years), 35 (76.1%) patients were able to discontinue corticosteroids, 11 (23.9%) patients were able to taper to <7.5 mg/day and only 1 (2.2%) patient required 10 mg of prednisone. The mean visual acuity at the latest follow-up of the worse eye was logarithm of the minimum angle of resolution (logMAR) 0.42 (SD 0.72), while the mean visual acuity of the better eye was logMAR 0.19 (SD 0.34). Of the 89 eyes, 21 (23.6%) eyes improved by at least 2 lines, 5 eyes (5.6%) deteriorated by ≥2 lines while vision was unchanged in the remaining 63 (70.8%) eyes. The time to recurrence was 1 in 12.47 person-years for adalimumab, with a 17.4% (8 patient) relapse rate. There were no serious adverse events.
Conclusions: This study highlights the efficacy of adalimumab in patients with vision-threatening non-infectious uveitis, preserving vision and allowing reduction of corticosteroid dose.
Keywords: drugs; immunology; inflammation; vision.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
LinkOut - more resources
Full Text Sources