Epilepsy in the mTORopathies: opportunities for precision medicine
- PMID: 34632383
- PMCID: PMC8495134
- DOI: 10.1093/braincomms/fcab222
Epilepsy in the mTORopathies: opportunities for precision medicine
Abstract
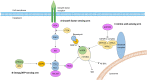
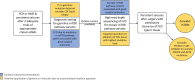
The mechanistic target of rapamycin signalling pathway serves as a ubiquitous regulator of cell metabolism, growth, proliferation and survival. The main cellular activity of the mechanistic target of rapamycin cascade funnels through mechanistic target of rapamycin complex 1, which is inhibited by rapamycin, a macrolide compound produced by the bacterium Streptomyces hygroscopicus. Pathogenic variants in genes encoding upstream regulators of mechanistic target of rapamycin complex 1 cause epilepsies and neurodevelopmental disorders. Tuberous sclerosis complex is a multisystem disorder caused by mutations in mechanistic target of rapamycin regulators TSC1 or TSC2, with prominent neurological manifestations including epilepsy, focal cortical dysplasia and neuropsychiatric disorders. Focal cortical dysplasia type II results from somatic brain mutations in mechanistic target of rapamycin pathway activators MTOR, AKT3, PIK3CA and RHEB and is a major cause of drug-resistant epilepsy. DEPDC5, NPRL2 and NPRL3 code for subunits of the GTPase-activating protein (GAP) activity towards Rags 1 complex (GATOR1), the principal amino acid-sensing regulator of mechanistic target of rapamycin complex 1. Germline pathogenic variants in GATOR1 genes cause non-lesional focal epilepsies and epilepsies associated with malformations of cortical development. Collectively, the mTORopathies are characterized by excessive mechanistic target of rapamycin pathway activation and drug-resistant epilepsy. In the first large-scale precision medicine trial in a genetically mediated epilepsy, everolimus (a synthetic analogue of rapamycin) was effective at reducing seizure frequency in people with tuberous sclerosis complex. Rapamycin reduced seizures in rodent models of DEPDC5-related epilepsy and focal cortical dysplasia type II. This review outlines a personalized medicine approach to the management of epilepsies in the mTORopathies. We advocate for early diagnostic sequencing of mechanistic target of rapamycin pathway genes in drug-resistant epilepsy, as identification of a pathogenic variant may point to an occult dysplasia in apparently non-lesional epilepsy or may uncover important prognostic information including, an increased risk of sudden unexpected death in epilepsy in the GATORopathies or favourable epilepsy surgery outcomes in focal cortical dysplasia type II due to somatic brain mutations. Lastly, we discuss the potential therapeutic application of mechanistic target of rapamycin inhibitors for drug-resistant seizures in GATOR1-related epilepsies and focal cortical dysplasia type II.
Keywords: GATOR1-related epilepsies; everolimus; focal cortical dysplasia type II; the mTORopathies; tuberous sclerosis complex.
© The Author(s) (2021). Published by Oxford University Press on behalf of the Guarantors of Brain.
Figures
Similar articles
-
Involvement of GATOR complex genes in familial focal epilepsies and focal cortical dysplasia.Epilepsia. 2016 Jun;57(6):994-1003. doi: 10.1111/epi.13391. Epub 2016 May 13. Epilepsia. 2016. PMID: 27173016
-
mTOR pathway: Insights into an established pathway for brain mosaicism in epilepsy.Neurobiol Dis. 2023 Jun 15;182:106144. doi: 10.1016/j.nbd.2023.106144. Epub 2023 May 4. Neurobiol Dis. 2023. PMID: 37149062 Review.
-
GATORopathies: The role of amino acid regulatory gene mutations in epilepsy and cortical malformations.Epilepsia. 2019 Nov;60(11):2163-2173. doi: 10.1111/epi.16370. Epub 2019 Oct 17. Epilepsia. 2019. PMID: 31625153 Free PMC article. Review.
-
DEPDC5 as a potential therapeutic target for epilepsy.Expert Opin Ther Targets. 2017 Jun;21(6):591-600. doi: 10.1080/14728222.2017.1316715. Epub 2017 Apr 13. Expert Opin Ther Targets. 2017. PMID: 28406046 Review.
-
GATOR1 complex: the common genetic actor in focal epilepsies.J Med Genet. 2016 Aug;53(8):503-10. doi: 10.1136/jmedgenet-2016-103883. Epub 2016 May 19. J Med Genet. 2016. PMID: 27208208 Review.
Cited by
-
The Genetics of Tuberous Sclerosis Complex and Related mTORopathies: Current Understanding and Future Directions.Genes (Basel). 2024 Mar 4;15(3):332. doi: 10.3390/genes15030332. Genes (Basel). 2024. PMID: 38540392 Free PMC article. Review.
-
Structures and Functions of the Human GATOR1 Complex.Subcell Biochem. 2024;104:269-294. doi: 10.1007/978-3-031-58843-3_12. Subcell Biochem. 2024. PMID: 38963491 Free PMC article. Review.
-
Surgical decision-making in adult patients with epilepsy related to germline mutations: A single-center study.J Int Med Res. 2025 May;53(5):3000605251342517. doi: 10.1177/03000605251342517. Epub 2025 May 24. J Int Med Res. 2025. PMID: 40411393 Free PMC article.
-
Efficacy and safety of cannabidiol in a single-center pediatric drug-resistant epilepsy cohort: a retrospective study.Front Neurol. 2025 Jul 16;16:1616480. doi: 10.3389/fneur.2025.1616480. eCollection 2025. Front Neurol. 2025. PMID: 40740850 Free PMC article.
-
Tsc1 Haploinsufficiency Leads to Pax2 Dysregulation in the Developing Murine Cerebellum.Front Mol Neurosci. 2022 May 13;15:831687. doi: 10.3389/fnmol.2022.831687. eCollection 2022. Front Mol Neurosci. 2022. PMID: 35645731 Free PMC article.
References
-
- Helbig KL, Farwell Hagman KD, Shinde DN, et al.Diagnostic exome sequencing provides a molecular diagnosis for a significant proportion of patients with epilepsy. Genet Med. 2016;18(9):898–905. - PubMed
-
- Krenn M, Wagner M, Hotzy C, et al.Diagnostic exome sequencing in non-acquired focal epilepsies highlights a major role of GATOR1 complex genes. J Med Genet. 2020;57(9):624–633. - PubMed
-
- Ricos MG, Hodgson BL, Pippucci T, et al.; Epilepsy Electroclinical Study Group. Mutations in the mammalian target of rapamycin pathway regulators NPRL2 and NPRL3 cause focal epilepsy. Ann Neurol. 2016;79(1):120–131. - PubMed
-
- Kearney H, Byrne S, Cavalleri GL, Delanty N.. Tackling epilepsy with high-definition precision medicine: A review. JAMA Neurol. 2019;76(9):1109–1116. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
