Comparison of 68Ga-labeled Prostate-specific Membrane Antigen Ligand Positron Emission Tomography/Magnetic Resonance Imaging and Positron Emission Tomography/Computed Tomography for Primary Staging of Prostate Cancer: A Systematic Review and Meta-analysis
- PMID: 34632423
- PMCID: PMC8488242
- DOI: 10.1016/j.euros.2021.09.006
Comparison of 68Ga-labeled Prostate-specific Membrane Antigen Ligand Positron Emission Tomography/Magnetic Resonance Imaging and Positron Emission Tomography/Computed Tomography for Primary Staging of Prostate Cancer: A Systematic Review and Meta-analysis
Abstract
Context: In December 2020, the US Food and Drug Administration approved a 68Ga-labeled prostate-specific membrane antigen ligand (68Ga-PSMA-11) for positron emission tomography (PET) in patients with suspected prostate cancer (PCa) metastasis who are candidates for initial definitive therapy. 68Ga-PSMA PET is increasingly performed for these patients and is usually combined with computed tomography (CT). In recent years, 68Ga-PSMA PET has been combined with high-resolution magnetic resonance imaging (MRI), which is beneficial for T staging and may further enhance the staging of primary PCa.
Objective: To compare the diagnostic accuracy of 68Ga-PSMA PET/MRI with 68Ga-PSMA PET/CT for staging of primary PCa.
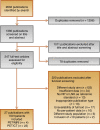
Evidence acquisition: A comprehensive literature search was performed using Embase, PubMed/Medline, Web of Science, Cochrane Library, and Google Scholar up to June 24, 2021 in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. Risk of bias was assessed using the QUADAS-2 tool.
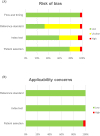
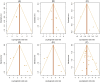
Evidence synthesis: The search identified 2632 articles, of which 27 were included. The diagnostic accuracy of 68Ga-PSMA PET/MRI, measured as the pooled natural logarithm of diagnostic odds ratio (lnDOR), was 2.27 (95% confidence interval [CI] 1.21-3.32) for detection of extracapsular extension (ECE), 3.50 (95% CI 2.14-4.86) for seminal vesicle invasion (SVI), and 4.73 (95% CI 2.93-6.52) for lymph node metastasis (LNM). For 68Ga-PSMA PET/CT, the analysis showed lnDOR of 2.45 (95% CI 0.75-4.14), 2.94 (95% CI 2.26-3.63), and 2.42 (95% CI 2.07-2.78) for detection of ECE, SVI, and LNM, respectively. The overall risk of bias and applicability concerns were assessed as moderate and low, respectively.
Conclusions: 68Ga-PSMA PET/MRI shows high diagnostic accuracy equivalent to that of 68Ga-PSMA PET/CT for detection of ECE, SVI, and LNM in staging of PCa. There is an urgent need for direct comparison of the two diagnostic tests in future research.
Patient summary: The use of radioactively labeled molecules that bind to prostate-specific membrane antigen (68Ga-PSMA) for positron emission tomography (PET) scans combined with either computed tomography (CT) or magnetic resonance imaging (MRI) is increasing for prostate cancer diagnosis. There is a need for direct comparison of the two tests to demonstrate the benefit of 68Ga-PSMA PET/MRI for determining tumor stage in prostate cancer.
Take home message: After the recent US Food and Drug Administration approval of 68Ga-labeled prostate-specific membrane antigen ligand (68Ga-PSMA) positron emission tomography (PET) for staging of primary prostate cancer (PCa), it is expected that the use of this imaging modality will increase rapidly. Our review of the literature shows that 68Ga-PSMA PET/magnetic resonance imaging has high diagnostic accuracy equivalent to that of 68Ga-PSMA PET/computed tomography in primary PCa staging. There is an urgent need for direct head-to-head comparison of the two diagnostic tests in future research.
Keywords: Diagnostic accuracy; Gallium-68; Positron emission tomography; Primary staging; Prostate cancer.
© 2021 The Authors.
Figures
References
-
- The Global Cancer Observatory. Prostate cancer factsheet. https://gco.iarc.fr/today/data/factsheets/cancers/27-Prostate-fact-sheet....
-
- Silver D.A., Pellicer I., Fair W.R., Heston W.D., Cordon-Cardo C. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin Cancer Res. 1997;3:81–85. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

