High-throughput assays show the timescale for phagocytic success depends on the target toughness
- PMID: 34632456
- PMCID: PMC8485781
- DOI: 10.1063/5.0057071
High-throughput assays show the timescale for phagocytic success depends on the target toughness
Abstract
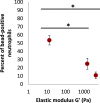
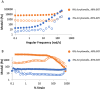
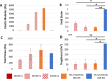
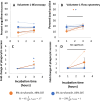
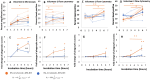
Phagocytic immune cells can clear pathogens from the body by engulfing them. Bacterial biofilms are communities of bacteria that are bound together in a matrix that gives biofilms viscoelastic mechanical properties that do not exist for free-swimming bacteria. Since a neutrophil is too small to engulf an entire biofilm, it must be able to detach and engulf a few bacteria at a time if it is to use phagocytosis to clear the infection. We recently found a negative correlation between the target elasticity and phagocytic success. That earlier work used time-consuming, manual analysis of micrographs of neutrophils and fluorescent beads. Here, we introduce and validate flow cytometry as a fast and high-throughput technique that increases the number of neutrophils analyzed per experiment by two orders of magnitude, while also reducing the time required to do so from hours to minutes. We also introduce the use of polyacrylamide gels in our assay for engulfment success. The tunability of polyacrylamide gels expands the mechanical parameter space we can study, and we find that high toughness and yield strain, even with low elasticity, also impact the phagocytic success as well as the timescale thereof. For stiff gels with low-yield strain, and consequent low toughness, phagocytic success is nearly four times greater when neutrophils are incubated with gels for 6 h than after only 1 h of incubation. In contrast, for soft gels with high-yield strain and consequent high toughness, successful engulfment is much less time-sensitive, increasing by less than a factor of two from 1 to 6 h incubation.
© 2021 Author(s).
Figures
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
