Recent advances in understanding the role of proteostasis
- PMID: 34632458
- PMCID: PMC8483240
- DOI: 10.12703/r/10-72
Recent advances in understanding the role of proteostasis
Abstract
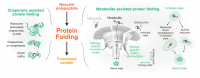
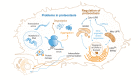
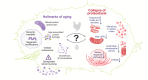
Maintenance of a functional proteome is achieved through the mechanism of proteostasis that involves precise coordination between molecular machineries assisting a protein from its conception to demise. Although each organelle within a cell has its own set of proteostasis machinery, inter-organellar communication and cell non-autonomous signaling bring forth the multidimensional nature of the proteostasis network. Exposure to extrinsic and intrinsic stressors can challenge the proteostasis network, leading to the accumulation of aberrant proteins or a decline in the proteostasis components, as seen during aging and in several diseases. Here, we summarize recent advances in understanding the role of proteostasis and its regulation in aging and disease, including monogenetic and infectious diseases. We highlight some of the emerging as well as unresolved questions in proteostasis that need to be addressed to overcome pathologies associated with damaged proteins and to promote healthy aging.
Keywords: Proteostasis; Unfolded Protein Response; aging; autophagy; metabolism; molecular chaperones; neurodegeneration; protein degradation; protein folding.
Copyright: © 2021 Chakraborty K et al.
Conflict of interest statement
The authors declare that they have no competing interests.No competing interests were disclosed.No competing interests were disclosed.No competing interests were disclosed.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
