The alarmin S100A12 causes sterile inflammation of the human chorioamniotic membranes as well as preterm birth and neonatal mortality in mice†
- PMID: 34632484
- PMCID: PMC8689293
- DOI: 10.1093/biolre/ioab188
The alarmin S100A12 causes sterile inflammation of the human chorioamniotic membranes as well as preterm birth and neonatal mortality in mice†
Abstract
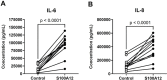
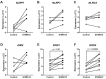
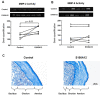
Sterile inflammation is triggered by danger signals, or alarmins, released upon cellular stress or necrosis. Sterile inflammation occurring in the amniotic cavity (i.e. sterile intra-amniotic inflammation) is frequently observed in women with spontaneous preterm labor resulting in preterm birth, the leading cause of neonatal morbidity and mortality worldwide; this condition is associated with increased amniotic fluid concentrations of alarmins. However, the mechanisms whereby alarmins induce sterile intra-amniotic inflammation are still under investigation. Herein, we investigated the mechanisms whereby the alarmin S100A12 induces inflammation of the human chorioamniotic membranes in vitro and used a mouse model to establish a causal link between this alarmin and adverse perinatal outcomes. We report that S100A12 initiates sterile inflammation in the chorioamniotic membranes by upregulating the expression of inflammatory mediators such as pro-inflammatory cytokines and pattern recognition receptors. Importantly, S100A12 induced the priming and activation of inflammasomes, resulting in caspase-1 cleavage and the subsequent release of mature IL-1β by the chorioamniotic membranes. This alarmin also caused the activation of the chorioamniotic membranes by promoting MMP-2 activity and collagen degradation. Lastly, the ultrasound-guided intra-amniotic injection of S100A12 at specific concentrations observed in the majority of women with sterile intra-amniotic inflammation induced preterm birth (rates: 17% at 200 ng/sac; 25% at 300 ng/sac; 25% at 400 ng/sac) and neonatal mortality (rates: 22% at 200 ng/sac; 44% at 300 ng/sac; 31% at 400 ng/sac), thus demonstrating a causal link between this alarmin and adverse perinatal outcomes. Collectively, our findings shed light on the inflammatory responses driven by alarmins in the chorioamniotic membranes, providing insight into the immune mechanisms leading to preterm birth in women with sterile intra-amniotic inflammation.
Keywords: Amniotic cavity; NLRP3; calgranulin C; caspase-1; chorioamnionitis; decidua; fetal membranes; funisitis; inflammasome; interleukin-1β; matrix metalloproteinase-2; pregnancy.
© The Author(s) 2021. Published by Oxford University Press on behalf of Society for the Study of Reproduction. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures


Similar articles
-
The alarmin interleukin-1α causes preterm birth through the NLRP3 inflammasome.Mol Hum Reprod. 2020 Sep 1;26(9):712-726. doi: 10.1093/molehr/gaaa054. Mol Hum Reprod. 2020. PMID: 32647859 Free PMC article.
-
Inhibition of the NLRP3 inflammasome can prevent sterile intra-amniotic inflammation, preterm labor/birth, and adverse neonatal outcomes†.Biol Reprod. 2019 May 1;100(5):1306-1318. doi: 10.1093/biolre/ioy264. Biol Reprod. 2019. PMID: 30596885 Free PMC article.
-
HMGB1 Induces an Inflammatory Response in the Chorioamniotic Membranes That Is Partially Mediated by the Inflammasome.Biol Reprod. 2016 Dec;95(6):130. doi: 10.1095/biolreprod.116.144139. Epub 2016 Nov 2. Biol Reprod. 2016. PMID: 27806943 Free PMC article.
-
The immunobiology of preterm labor and birth: intra-amniotic inflammation or breakdown of maternal-fetal homeostasis.Reproduction. 2022 Jun 20;164(2):R11-R45. doi: 10.1530/REP-22-0046. Reproduction. 2022. PMID: 35559791 Free PMC article. Review.
-
Acute chorioamnionitis and funisitis: definition, pathologic features, and clinical significance.Am J Obstet Gynecol. 2015 Oct;213(4 Suppl):S29-52. doi: 10.1016/j.ajog.2015.08.040. Am J Obstet Gynecol. 2015. PMID: 26428501 Free PMC article. Review.
Cited by
-
CKS2 and S100A12: Two Novel Diagnostic Biomarkers for Rheumatoid Arthritis.Dis Markers. 2022 Jun 25;2022:2431976. doi: 10.1155/2022/2431976. eCollection 2022. Dis Markers. 2022. Retraction in: Dis Markers. 2023 Jul 19;2023:9871012. doi: 10.1155/2023/9871012. PMID: 35789606 Free PMC article. Retracted.
-
Buprenorphine induces human fetal membrane sterile inflammation.J Reprod Immunol. 2025 Mar;168:104445. doi: 10.1016/j.jri.2025.104445. Epub 2025 Feb 1. J Reprod Immunol. 2025. PMID: 39914058
-
Clarithromycin prevents preterm birth and neonatal mortality by dampening alarmin-induced maternal-fetal inflammation in mice.BMC Pregnancy Childbirth. 2022 Jun 20;22(1):503. doi: 10.1186/s12884-022-04764-2. BMC Pregnancy Childbirth. 2022. PMID: 35725425 Free PMC article.
-
Arrest of mouse preterm labor until term delivery by combination therapy with atosiban and mundulone, a natural product with tocolytic efficacy.Pharmacol Res. 2023 Sep;195:106876. doi: 10.1016/j.phrs.2023.106876. Epub 2023 Aug 1. Pharmacol Res. 2023. PMID: 37536638 Free PMC article.
-
Intra-amniotic inflammation in the mid-trimester of pregnancy is a risk factor for neuropsychological disorders in childhood.J Perinat Med. 2022 Sep 29;51(3):363-378. doi: 10.1515/jpm-2022-0255. Print 2023 Mar 28. J Perinat Med. 2022. PMID: 36173676 Free PMC article.
References
-
- Preterm Birth: CDC ; 2021. https://www.cdc.gov/reproductivehealth/maternalinfanthealth/pretermbirth...; Accessed 16 October 2021.
-
- Scheuchenegger A, Lechner E, Wiesinger-Eidenberger G, Weissensteiner M, Wagner O, Schimetta W, Resch B. Short-term morbidities in moderate and late preterm infants. Klin Padiatr 2014; 226:216–220. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

