Measuring respiratory symptoms in moderate/severe asthma: evaluation of a respiratory symptom tool, the E-RS®: COPD in asthma populations
- PMID: 34632556
- PMCID: PMC8502721
- DOI: 10.1186/s41687-021-00338-6
Measuring respiratory symptoms in moderate/severe asthma: evaluation of a respiratory symptom tool, the E-RS®: COPD in asthma populations
Abstract
Background: Symptom constructs included in the Evaluating Respiratory Symptoms in Chronic Obstructive Pulmonary Disease (E-RS®: COPD) tool may be relevant to patients with asthma. The purpose of this study was to evaluate content validity and psychometric performance of the E-RS: COPD in moderate/severe asthma patients.
Methods: Content validity of the E-RS: COPD was evaluated in patients with moderate/severe asthma using concept elicitation and cognitive debriefing interviews. Secondary analyses using data from two clinical trials in patients with moderate/severe asthma evaluated the factor structure of the E-RS: COPD plus two supplementary items (wheeze; shortness of breath with strenuous physical activity) and assessed psychometric properties of the tool, which will be referred to as E-RS®: Asthma when used in asthma populations.
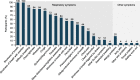
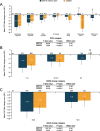
Results: Qualitative interviews (N = 25) achieved concept saturation for asthma respiratory symptoms. Concepts in the E-RS: COPD were relevant to patients and instructions were understood. Most patients (19/25; 76%) reported experiencing all concepts in the E-RS: COPD; no patients indicated missing symptoms. Secondary analyses of clinical trial data supported the original factor structure (RS-Total and three symptom-specific subscales). The two supplemental items did not fit with this factor structure and were not retained. RS-Total and subscale score reliability was high (internal consistency [α] > 0.70). Validity was demonstrated through significant (P < 0.0001) relationships with the St George's Respiratory Questionnaire (SGRQ) and Asthma Symptom Severity scale. E-RS: Asthma was responsive to change when evaluated using SGRQ, Patient Global Impression of Change and Asthma Quality of Life Questionnaire as anchors (P < 0.0001). Clinically meaningful change thresholds were also identified (RS-Total: - 2.0 units).
Conclusions: The E-RS: Asthma is reliable and responsive for evaluating respiratory symptoms in patients with moderate/severe asthma.
Keywords: Asthma; Content validity; Patient-reported outcome; Psychometric; Qualitative; Reliability; Respiratory symptoms; Responsiveness; Validity.
© 2021. The Author(s).
Conflict of interest statement
RvM, ZB, and AF are employees of and hold stock in GlaxoSmithKline (GSK). EDB, TAH, HK, and RH are employees of Evidera. Evidera provides consulting and other research services to pharmaceutical, device, government and non-government organizations. As Evidera employees, they work with a variety of companies and organizations and are expressly prohibited from receiving any payment or honoraria directly from these organizations for services rendered. LTM was a former employee of Evidera. MT and LL were former employees of GSK at the time this work was conducted and holds stocks or shares in GSK.
Figures

References
-
- Global Initiative for Asthma (2020) Global strategy for asthma management and prevention. https://ginasthma.org/. Accessed May 2020.
-
- Juniper EF. Assessing asthma quality of life: Its role in clinical practice. Breathe. 2005;1(3):192–204. doi: 10.1183/18106838.0103.192. - DOI
-
- Leidy NK, Wilcox TK, Jones PW, Roberts L, Powers JH, Sethi S. Standardizing measurement of chronic obstructive pulmonary disease exacerbations. Reliability and validity of a patient-reported diary. American Journal of Respiratory and Critical Care Medicine. 2011;183(3):323–329. doi: 10.1164/rccm.201005-0762OC. - DOI - PubMed
LinkOut - more resources
Full Text Sources

