CoCrMo-Nanoparticles induced peri-implant osteolysis by promoting osteoblast ferroptosis via regulating Nrf2-ARE signalling pathway
- PMID: 34632658
- PMCID: PMC8666286
- DOI: 10.1111/cpr.13142
CoCrMo-Nanoparticles induced peri-implant osteolysis by promoting osteoblast ferroptosis via regulating Nrf2-ARE signalling pathway
Abstract
Objectives: Aseptic loosening (AL) is the most common reason of total hip arthroplasty (THA) failure and revision surgery. Osteolysis, caused by wear particles released from implant surfaces, has a vital role in AL. Although previous studies suggest that wear particles always lead to osteoblast programmed death in the process of AL, the specific mechanism remains incompletely understood and osteoblast ferroptosis maybe a new mechanism of AL.
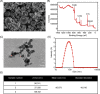
Materials and methods: CoCrMo nanoparticles (CoNPs) were prepared to investigate the influence of ferroptosis in osteoblasts and calvaria resorption animal models. Periprosthetic osteolytic bone tissue was collected from patients who underwent AL after THA to verify osteoblast ferroptosis.
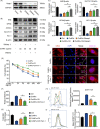
Results: Our study demonstrated that CoNPs induced significant ferroptosis in osteoblasts and particles induced osteolysis (PIO) animal models. Blocking ferroptosis with specific inhibitor Ferrostatin-1 dramatically reduced particle-induced ferroptosis in vitro. Moreover, in osteoblasts, CoNPs significantly downregulated the expression of Nrf2 (nuclear factor erythroid 2-related factor 2), a core element in the antioxidant response. The overexpression of Nrf2 by siKeap1 or Nrf2 activator Oltipraz obviously upregulated antioxidant response elements (AREs) and suppressed ferroptosis in osteoblasts. Furthermore, in PIO animal models, the combined utilization of Ferrostatin-1 and Oltipraz dramatically ameliorated ferroptosis and the severity of osteolysis.
Conclusions: These results indicate that CoNPs promote osteoblast ferroptosis by regulating the Nrf2-ARE signalling pathway, which suggests a new mechanism underlying PIO and represents a potential therapeutic approach for AL.
© 2021 The Authors. Cell Proliferation published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest for this work.
Figures







References
-
- Sheth NP, Rozell JC, Paprosky WG. Evaluation and treatment of patients with acetabular osteolysis after total hip arthroplasty. J Am Acad Orthop Surg. 2019;27(6):e258‐e267. - PubMed
-
- Pivec R, Johnson AJ, Mears SC, Mont MA. Hip arthroplasty. Lancet. 2012;380(9855):1768‐1777. - PubMed
-
- Rames RD, Hillen TJ, Pashos GE, Maloney WJ, Clohisy JC. Incidence and characteristics of osteolysis in HXLPE THA at 16‐year follow up in patients 50 years and less. J Arthroplasty. 2021;36(2):641‐646. - PubMed
MeSH terms
Substances
Grants and funding
- 31771048/National Natural Science Foundation of China
- 81871795/National Natural Science Foundation of China
- 18sjkjgg18/Songjiang District Science and Technology Research project
- SHDC12017121/Shanghai Municipal Health and Family Planning Commission
- YG2019QNB37/Medical Engineering Cross Research Project of Shanghai Jiaotong University
LinkOut - more resources
Full Text Sources

