Hydrogen Bonding and Vaporization Thermodynamics in Hexafluoroisopropanol-Acetone and -Methanol Mixtures. A Joined Cluster Analysis and Molecular Dynamic Study
- PMID: 34632686
- PMCID: PMC9298724
- DOI: 10.1002/cphc.202100620
Hydrogen Bonding and Vaporization Thermodynamics in Hexafluoroisopropanol-Acetone and -Methanol Mixtures. A Joined Cluster Analysis and Molecular Dynamic Study
Abstract
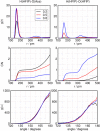
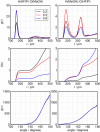
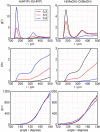
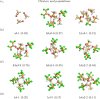
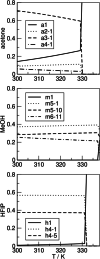
Binary mixtures of hexafluoroisopropanol with either methanol or acetone are analyzed via classical molecular dynamics simulations and quantum cluster equilibrium calculations. In particular, their populations and thermodynamic properties are investigated with the binary quantum cluster equilibrium method, using our in-house code Peacemaker 2.8, upgraded with temperature-dependent parameters. A novel approach, where the final density from classical molecular dynamics, has been used to generate the necessary reference isobars. The hydrogen bond network in both type of mixtures at molar fraction of hexafluoroisopropanol of 0.2, 0.5, and 0.8 respectively is investigated via the molecular dynamics trajectories and the cluster results. In particular, the populations show that mixed clusters are preferred in both systems even at 0.2 molar fractions of hexafluoroisopropanol. Enthalpies and entropies of vaporization are calculated for the neat and mixed systems and found to be in good agreement with experimental values.
Keywords: enthalpy of vaporization; hexafluoroisopropanol; mixtures; molecular dynamics simulations; quantum cluster equilibrium.
© 2021 The Authors. ChemPhysChem published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Activity coefficients of binary methanol alcohol mixtures from cluster weighting.ChemistryOpen. 2020 Jul 23;9(7):774-785. doi: 10.1002/open.202000171. eCollection 2020 Jul. ChemistryOpen. 2020. PMID: 32714740 Free PMC article.
-
Structural properties of methanol-water binary mixtures within the quantum cluster equilibrium model.Phys Chem Chem Phys. 2015 Apr 7;17(13):8467-79. doi: 10.1039/c4cp05836d. Epub 2015 Feb 9. Phys Chem Chem Phys. 2015. PMID: 25660666
-
Properties of the liquid-vapor interface of acetone-methanol mixtures, as seen from computer simulation and ITIM surface analysis.Phys Chem Chem Phys. 2015 Apr 14;17(14):8913-26. doi: 10.1039/c4cp05974c. Epub 2015 Mar 6. Phys Chem Chem Phys. 2015. PMID: 25746419
-
Ab initio calculations of thermochemical properties of methanol clusters.J Phys Chem A. 2013 Feb 21;117(7):1569-82. doi: 10.1021/jp308908j. Epub 2013 Feb 8. J Phys Chem A. 2013. PMID: 23330733
-
Detailed insight into the hydrogen bonding interactions in acetone-methanol mixtures. A molecular dynamics simulation and Voronoi polyhedra analysis study.Phys Chem Chem Phys. 2012 May 7;14(17):5979-87. doi: 10.1039/c2cp24101c. Epub 2012 Mar 23. Phys Chem Chem Phys. 2012. PMID: 22446731
Cited by
-
The Ionic Product of Water in the Eye of the Quantum Cluster Equilibrium.Molecules. 2022 Feb 14;27(4):1286. doi: 10.3390/molecules27041286. Molecules. 2022. PMID: 35209075 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

