Conquering the cytokine storm in COVID-19-induced ARDS using placenta-derived decidua stromal cells
- PMID: 34632708
- PMCID: PMC8581334
- DOI: 10.1111/jcmm.16986
Conquering the cytokine storm in COVID-19-induced ARDS using placenta-derived decidua stromal cells
Abstract
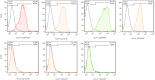
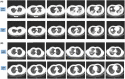
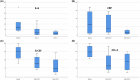
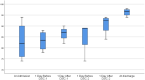
Acute respiratory distress syndrome (ARDS) is the most common cause of death in COVID-19 patients. The cytokine storm is the main driver of the severity and magnitude of ARDS. Placenta-derived decidua stromal cells (DSCs) have a stronger immunosuppressive effect than other sources of mesenchymal stromal cells. Safety and efficacy study included 10 patients with a median age of 50 (range 14-68) years with COVID-19-induced ARDS. DSCs were administered 1-2 times at a dose of 1 × 106 /kg. End points were safety and efficacy by survival, oxygenation and effects on levels of cytokines. Oxygenation levels increased from a median of 80.5% (range 69-88) to 95% (range 78-99) (p = 0.012), and pulmonary infiltrates disappeared in all patients. Levels of IL-6 decreased from a median of 69.3 (range 35.0-253.4) to 11 (range 4.0-38.3) pg/ml (p = 0.018), and CRP decreased from 69 (range 5-169) to 6 (range 2-31) mg/ml (p = 0.028). Two patients died, one of a myocardial infarction and the other of multiple organ failure, diagnosed before the DSC therapy. The other patients recovered and left the intensive care unit (ICU) within a median of 6 (range 3-12) days. DSC therapy is safe and capable of improving oxygenation, decreasing inflammatory cytokine level and clearing pulmonary infiltrates in patients with COVID-19.
Keywords: COVID-19; cytokine storm; decidua stromal cells; intensive care unit; survival.
© 2021 The Authors. Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
Dr Behnam Sadeghi is founder of Sibcell Biotech. All other authors declare no competing interests.
Figures
Similar articles
-
Mesenchymal stem cells derived from perinatal tissues for treatment of critically ill COVID-19-induced ARDS patients: a case series.Stem Cell Res Ther. 2021 Jan 29;12(1):91. doi: 10.1186/s13287-021-02165-4. Stem Cell Res Ther. 2021. PMID: 33514427 Free PMC article. Clinical Trial.
-
Double-blind, randomized, controlled, trial to assess the efficacy of allogenic mesenchymal stromal cells in patients with acute respiratory distress syndrome due to COVID-19 (COVID-AT): A structured summary of a study protocol for a randomised controlled trial.Trials. 2021 Jan 6;22(1):9. doi: 10.1186/s13063-020-04964-1. Trials. 2021. PMID: 33407777 Free PMC article.
-
Early Hemoperfusion for Cytokine Removal May Contribute to Prevention of Intubation in Patients Infected with COVID-19.Blood Purif. 2021;50(2):257-260. doi: 10.1159/000509107. Epub 2020 Jun 26. Blood Purif. 2021. PMID: 32594085 Free PMC article.
-
Mesenchymal stromal cells as treatment for acute respiratory distress syndrome. Case Reports following hematopoietic cell transplantation and a review.Front Immunol. 2022 Nov 8;13:963445. doi: 10.3389/fimmu.2022.963445. eCollection 2022. Front Immunol. 2022. PMID: 36426365 Free PMC article. Review.
-
Potential and challenges of placenta-derived decidua stromal cell therapy in inflammation-associated disorders.Hum Immunol. 2022 Jul;83(7):580-588. doi: 10.1016/j.humimm.2022.04.011. Epub 2022 May 28. Hum Immunol. 2022. PMID: 35637033 Review.
Cited by
-
Efficacy and Safety of MSC Cell Therapies for Hospitalized Patients with COVID-19: A Systematic Review and Meta-Analysis.Stem Cells Transl Med. 2022 Jul 20;11(7):688-703. doi: 10.1093/stcltm/szac032. Stem Cells Transl Med. 2022. PMID: 35640138 Free PMC article.
-
Advances in acute respiratory distress syndrome: focusing on heterogeneity, pathophysiology, and therapeutic strategies.Signal Transduct Target Ther. 2025 Mar 7;10(1):75. doi: 10.1038/s41392-025-02127-9. Signal Transduct Target Ther. 2025. PMID: 40050633 Free PMC article. Review.
-
The rise of exosome-mediated mechanisms in MSC therapy.J Transl Med. 2025 Jul 14;23(1):793. doi: 10.1186/s12967-025-06747-1. J Transl Med. 2025. PMID: 40660274 Free PMC article.
-
Mesenchymal Stromal Cells for Enhancing Hematopoietic Engraftment and Treatment of Graft-Versus-Host Disease, Hemorrhages and Acute Respiratory Distress Syndrome.Front Immunol. 2022 Mar 18;13:839844. doi: 10.3389/fimmu.2022.839844. eCollection 2022. Front Immunol. 2022. PMID: 35371003 Free PMC article.
-
Mesenchymal stem cells in treating human diseases: molecular mechanisms and clinical studies.Signal Transduct Target Ther. 2025 Aug 22;10(1):262. doi: 10.1038/s41392-025-02313-9. Signal Transduct Target Ther. 2025. PMID: 40841367 Free PMC article. Review.
References
-
- University, J.H . Coronavirus Resource Center. 2021; Available from: https://coronavirus.jhu.edu
-
- Murthy S, Gomersall CD, Fowler RA. Care for critically ill patients with COVID‐19. JAMA. 2020;323(15):1499‐1500. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

