Effects of propofol and its formulation components on macrophages and neutrophils in obese and lean animals
- PMID: 34632734
- PMCID: PMC8503301
- DOI: 10.1002/prp2.873
Effects of propofol and its formulation components on macrophages and neutrophils in obese and lean animals
Abstract
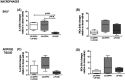
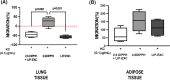
We hypothesized whether propofol or active propofol component (2,6-diisopropylphenol [DIPPH] and lipid excipient [LIP-EXC]) separately may alter inflammatory mediators expressed by macrophages and neutrophils in lean and obese rats. Male Wistar rats (n = 10) were randomly assigned to receive a standard (lean) or obesity-inducing diet (obese) for 12 weeks. Animals were euthanized, and alveolar macrophages and neutrophils from lean and obese animals were exposed to propofol (50 µM), active propofol component (50 µM, 2,6-DIPPH), and lipid excipient (soybean oil, purified egg phospholipid, and glycerol) for 1 h. The primary outcome was IL-6 expression after propofol and its components exposure by alveolar macrophages extracted from bronchoalveolar lavage fluid. The secondary outcomes were the production of mediators released by macrophages from adipose tissue, and neutrophils from lung and adipose tissues, and neutrophil migration. IL-6 increased after the exposure to both propofol (median [interquartile range] 4.14[1.95-5.20]; p = .04) and its active component (2,6-DIPPH) (4.09[1.67-5.91]; p = .04) in alveolar macrophages from obese animals. However, only 2,6-DIPPH increased IL-10 expression (7.59[6.28-12.95]; p = .001) in adipose tissue-derived macrophages. Additionally, 2,6-DIPPH increased C-X-C chemokine receptor 2 and 4 (CXCR2 and CXCR4, respectively) in lung (10.08[8.23-29.01]; p = .02; 1.55[1.49-3.43]; p = .02) and adipose tissues (8.78[4.15-11.57]; p = .03; 2.86[2.17-3.71]; p = .01), as well as improved lung-derived neutrophil migration (28.00[-3.42 to 45.07]; p = .001). In obesity, the active component of propofol affected both the M1 and M2 markers as well as neutrophils in both alveolar and adipose tissue cells, suggesting that lipid excipient may hinder the effects of active propofol.
Keywords: adipose tissue; inflammation; lung; macrophages; neutrophils; obesity; propofol.
© 2021 The Authors. Pharmacology Research & Perspectives published by British Pharmacological Society and American Society for Pharmacology and Experimental Therapeutics and John Wiley & Sons Ltd.
Conflict of interest statement
The authors have no conflict of interest.
Figures


Similar articles
-
Comparison between sevoflurane and propofol on immunomodulation in an in vitro model of sepsis.Front Med (Lausanne). 2023 Jul 27;10:1225179. doi: 10.3389/fmed.2023.1225179. eCollection 2023. Front Med (Lausanne). 2023. PMID: 37575989 Free PMC article.
-
Expression of genes for proinflammatory cytokines in alveolar macrophages during propofol and isoflurane anesthesia.Anesth Analg. 1999 Nov;89(5):1250-6. Anesth Analg. 1999. PMID: 10553845 Clinical Trial.
-
Effects of Obesity on Pulmonary Inflammation and Remodeling in Experimental Moderate Acute Lung Injury.Front Immunol. 2019 May 29;10:1215. doi: 10.3389/fimmu.2019.01215. eCollection 2019. Front Immunol. 2019. PMID: 31275296 Free PMC article.
-
CXCR2 antagonists block the N-Ac-PGP-induced neutrophil influx in the airways of mice, but not the production of the chemokine CXCL1.Eur J Pharmacol. 2011 Oct 15;668(3):443-9. doi: 10.1016/j.ejphar.2011.03.025. Epub 2011 Mar 31. Eur J Pharmacol. 2011. PMID: 21458445
-
Obesity is associated with neutrophil dysfunction and attenuation of murine acute lung injury.Am J Respir Cell Mol Biol. 2012 Jul;47(1):120-7. doi: 10.1165/rcmb.2011-0334OC. Epub 2012 Mar 15. Am J Respir Cell Mol Biol. 2012. PMID: 22427537 Free PMC article.
Cited by
-
Effects of propofol on macrophage activation and function in diseases.Front Pharmacol. 2022 Aug 17;13:964771. doi: 10.3389/fphar.2022.964771. eCollection 2022. Front Pharmacol. 2022. PMID: 36059940 Free PMC article. Review.
-
Comparison between sevoflurane and propofol on immunomodulation in an in vitro model of sepsis.Front Med (Lausanne). 2023 Jul 27;10:1225179. doi: 10.3389/fmed.2023.1225179. eCollection 2023. Front Med (Lausanne). 2023. PMID: 37575989 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

