Saturated fat ingestion stimulates proatherogenic inflammation in polycystic ovary syndrome
- PMID: 34632798
- PMCID: PMC8782660
- DOI: 10.1152/ajpendo.00213.2021
Saturated fat ingestion stimulates proatherogenic inflammation in polycystic ovary syndrome
Abstract
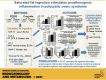
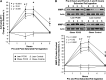
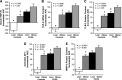
Inflammation and dyslipidemia are often present in polycystic ovary syndrome (PCOS). We determined the effect of saturated fat ingestion on circulating heat shock protein-70 (HSP-70) and mononuclear cell (MNC) toll-like receptor-2 (TLR2) gene expression, activator protein-1 (AP-1) activation, and matrix matalloproteinase-2 (MMP-2) protein in women with PCOS. Twenty reproductive-age women with PCOS (10 lean, 10 with obesity) and 20 ovulatory controls (10 lean, 10 with obesity) participated in the study. HSP-70 was measured in serum and TLR2 mRNA and protein, AP-1 activation, and MMP-2 protein were quantified in MNC from blood drawn while fasting and 2, 3, and 5 h after saturated fat ingestion. Insulin sensitivity was derived from an oral glucose tolerance test (ISOGTT). Androgen secretion was assessed from blood drawn while fasting and 24, 48, and 72 h after human chorionic gonadotropin (HCG) administration. In response to saturated fat ingestion, serum HSP-70, TLR2 gene expression, activated AP-1, and MMP-2 protein were greater in lean women with PCOS compared with lean controls and in women with PCOS and obesity compared with controls with obesity. Both PCOS groups exhibited lower ISOGTT and greater HCG-stimulated androgen secretion compared with control subjects of their respective weight classes. Lipid-stimulated proatherogenic inflammation marker responses were negatively correlated with ISOGTT and positively correlated with abdominal adiposity and HCG-stimulated androgen secretion. In PCOS, saturated fat ingestion stimulates proatherogenic inflammation independent of obesity. This effect is greater when PCOS is combined with obesity compared with obesity alone. Abdominal adiposity and hyperandrogenism may perpetuate proatherogenic inflammation.NEW & NOTEWORTHY This paper demonstrates that in polycystic ovary syndrome (PCOS), ingestion of saturated fat triggers a molecular pathway of inflammation known to drive atherogenesis. This effect is independent of obesity as it occurs in lean women with PCOS and not in lean ovulatory control subjects. Furthermore, the combined effects of PCOS and obesity are greater compared with obesity alone.
Keywords: abdominal adiposity; atherogenesis; hyperandrogenism; polycystic ovary syndrome; saturated fat.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures

References
-
- Fauser BCJM, Tarlatzis BC, Rebar RW, Legro RS, Balen AH, Lobo R, Carmina E, Chang J, Yildiz BO, Laven JSE, Boivin J, Petraglia F, Wijeyeratne CN, Norman RJ, Dunaif A, Franks S, Wild RA, Dumesic D, Barnhart K. Consensus on women’s health aspects of polycystic ovary syndrome (PCOS): the Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS Consensus Workshop Group. Fertil Steril 97: 28–38.e25, 2012. doi:10.1016/j.fertnstert.2011.09.024. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

