Epitope-specific anti-PrP antibody toxicity: a comparative in-silico study of human and mouse prion proteins
- PMID: 34632945
- PMCID: PMC8900626
- DOI: 10.1080/19336896.2021.1964326
Epitope-specific anti-PrP antibody toxicity: a comparative in-silico study of human and mouse prion proteins
Abstract
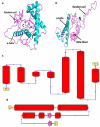
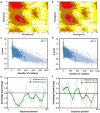
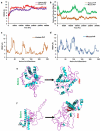
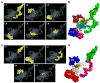
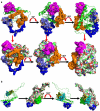
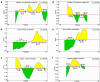
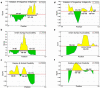
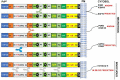
Despite having therapeutic potential, anti-PrP antibodies caused a major controversy due to their neurotoxic effects. For instance, treating mice with ICSM antibodies delayed prion disease onset, but both were found to be either toxic or innocuous to neurons by researchers following cross-linking PrPC. In order to elucidate and understand the reasons that led to these contradictory outcomes, we conducted a comprehensive in silico study to assess the antibody-specific toxicity. Since most therapeutic anti-PrP antibodies were generated against human truncated recombinant PrP91-231 or full-length mouse PrP23-231, we reasoned that host specificity (human vs murine) of PrPC might influence the nature of the specific epitopes recognized by these antibodies at the structural level possibly explaining the 'toxicity' discrepancies reported previously. Initially, molecular dynamics simulation and pro-motif analysis of full-length human (hu)PrP and mouse (mo)PrP 3D structure displayed conspicuous structural differences between huPrP and moPrP. We identified 10 huPrP and 6 moPrP linear B-cell epitopes from the prion protein 3D structure where 5 out of 10 huPrP and 3 out of 6 moPrP B-cell epitopes were predicted to be potentially toxic in immunoinformatics approaches. Herein, we demonstrate that some of the predicted potentially 'toxic' epitopes identified by the in silico analysis were similar to the epitopes recognized by the toxic antibodies such as ICSM18 (146-159), POM1 (138-147), D18 (133-157), ICSM35 (91-110), D13 (95-103) and POM3 (95-100). This in silico study reveals the role of host specificity of PrPC in epitope-specific anti-PrP antibody toxicity.
Keywords: B-cell epitope; Cellular prion protein (PrPc); immunoinformatics; molecular dynamics simulation; neurotoxicity.
Conflict of interest statement
The author(s) declare no competing interests.
Figures


Similar articles
-
Cross-Linking Cellular Prion Protein Induces Neuronal Type 2-Like Hypersensitivity.Front Immunol. 2021 Jul 30;12:639008. doi: 10.3389/fimmu.2021.639008. eCollection 2021. Front Immunol. 2021. PMID: 34394070 Free PMC article.
-
Structural studies on the folded domain of the human prion protein bound to the Fab fragment of the antibody POM1.Acta Crystallogr D Biol Crystallogr. 2012 Nov;68(Pt 11):1501-12. doi: 10.1107/S0907444912037328. Epub 2012 Oct 18. Acta Crystallogr D Biol Crystallogr. 2012. PMID: 23090399
-
Mouse prion protein (PrP) segment 100 to 104 regulates conversion of PrP(C) to PrP(Sc) in prion-infected neuroblastoma cells.J Virol. 2012 May;86(10):5626-36. doi: 10.1128/JVI.06606-11. Epub 2012 Mar 7. J Virol. 2012. PMID: 22398286 Free PMC article.
-
Prion protein transgenes and the neuropathology in prion diseases.Brain Pathol. 1995 Jan;5(1):77-89. doi: 10.1111/j.1750-3639.1995.tb00579.x. Brain Pathol. 1995. PMID: 7767493 Review.
-
Prion neurotoxicity.Brain Pathol. 2019 Mar;29(2):263-277. doi: 10.1111/bpa.12694. Epub 2019 Jan 17. Brain Pathol. 2019. PMID: 30588688 Free PMC article. Review.
References
-
- Prusiner SB. Novel proteinaceous infectious particles cause scrapie. Science. 1982;216(4542):136–144. - PubMed
-
- Baskakov IV, Breydo L, Converting the prion protein: what makes the protein infectious. Biochim Biophys Acta - Mol Basis Dis. 2007; 1772(6):692–703. - PubMed
-
- Liao YC, Lebo RV, Clawson GA, et al., Human prion protein cDNA: molecular cloning, chromosomal mapping, and biological implications. Science. 1986; 233(4761):364–367. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
