A beta-glucosidase of an insect herbivore determines both toxicity and deterrence of a dandelion defense metabolite
- PMID: 34632981
- PMCID: PMC8504966
- DOI: 10.7554/eLife.68642
A beta-glucosidase of an insect herbivore determines both toxicity and deterrence of a dandelion defense metabolite
Abstract
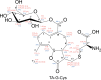
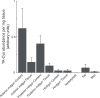
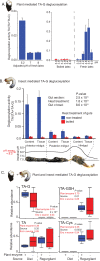
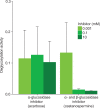
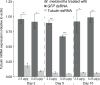
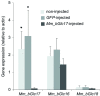
Gut enzymes can metabolize plant defense compounds and thereby affect the growth and fitness of insect herbivores. Whether these enzymes also influence feeding preference is largely unknown. We studied the metabolization of taraxinic acid β-D-glucopyranosyl ester (TA-G), a sesquiterpene lactone of the common dandelion (Taraxacum officinale) that deters its major root herbivore, the common cockchafer larva (Melolontha melolontha). We have demonstrated that TA-G is rapidly deglucosylated and conjugated to glutathione in the insect gut. A broad-spectrum M. melolontha β-glucosidase, Mm_bGlc17, is sufficient and necessary for TA-G deglucosylation. Using cross-species RNA interference, we have shown that Mm_bGlc17 reduces TA-G toxicity. Furthermore, Mm_bGlc17 is required for the preference of M. melolontha larvae for TA-G-deficient plants. Thus, herbivore metabolism modulates both the toxicity and deterrence of a plant defense compound. Our work illustrates the multifaceted roles of insect digestive enzymes as mediators of plant-herbivore interactions.
Keywords: Melolontha melolontha; Taraxacum officinale; ecology; plant defense; root herbivore; sesquiterpene lactone; β-glucosidase.
Plain language summary
Plants produce certain substances to fend off attackers like plant-feeding insects. To stop these compounds from damaging their own cells, plants often attach sugar molecules to them. When an insect tries to eat the plant, the plant removes the stabilizing sugar, ‘activating’ the compounds and making them toxic or foul-tasting. Curiously, some insects remove the sugar themselves, but it is unclear what consequences this has, especially for insect behavior. Dandelions, Taraxacum officinale, make high concentrations of a sugar-containing defense compound in their roots called taraxinic acid β-D-glucopyranosyl ester, or TA-G for short. TA-G deters the larvae of the Maybug – a pest also known as the common cockchafer or the doodlebug – from eating dandelion roots. When Maybug larvae do eat TA-G, it is found in their systems without its sugar. However, it is unclear whether it is the plant or the larva that removes the sugar. A second open question is how the sugar removal process affects the behavior of the Maybug larvae. Using chemical analysis and genetic manipulation, Huber et al. investigated what happens when Maybug larvae eat TA-G. This revealed that the acidity levels in the larvae’s digestive system deactivate the proteins from the dandelion that would normally remove the sugar from TA-G. However, rather than leaving the compound intact, larvae remove the sugar from TA-G themselves. They do this using a digestive enzyme, known as a beta-glucosidase, that cuts through sugar. Removing the sugar from TA-G made the compound less toxic, allowing the larvae to grow bigger, but it also increased TA-G’s deterrent effects, making the larvae less likely to eat the roots. Any organism that eats plants, including humans, must deal with chemicals like TA-G in their food. Once inside the body, enzymes can change these chemicals, altering their effects. This happens with many medicines, too. In the future, it might be possible to design compounds that activate only in certain species, or under certain conditions. Further studies in different systems may aid the development of new methods of pest control, or new drug treatments.
© 2021, Huber et al.
Conflict of interest statement
MH, TR, SI, AR, SG, JF, PR, MR, CP, NL, LH, ZB, YM, WH, CR, JG, ME No competing interests declared
Figures









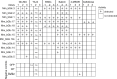
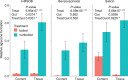
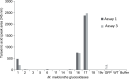

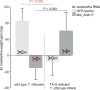



Similar articles
-
A Latex Metabolite Benefits Plant Fitness under Root Herbivore Attack.PLoS Biol. 2016 Jan 5;14(1):e1002332. doi: 10.1371/journal.pbio.1002332. eCollection 2016 Jan. PLoS Biol. 2016. PMID: 26731567 Free PMC article.
-
Impact of Seasonal and Temperature-Dependent Variation in Root Defense Metabolites on Herbivore Preference in Taraxacum officinale.J Chem Ecol. 2020 Jan;46(1):63-75. doi: 10.1007/s10886-019-01126-9. Epub 2019 Dec 12. J Chem Ecol. 2020. PMID: 31832894 Free PMC article.
-
A Herbivore Tag-and-Trace System Reveals Contact- and Density-Dependent Repellence of a Root Toxin.J Chem Ecol. 2017 Mar;43(3):295-306. doi: 10.1007/s10886-017-0830-3. Epub 2017 Mar 16. J Chem Ecol. 2017. PMID: 28303526
-
The potential of dandelion in the fight against gastrointestinal diseases: A review.J Ethnopharmacol. 2022 Jul 15;293:115272. doi: 10.1016/j.jep.2022.115272. Epub 2022 Apr 8. J Ethnopharmacol. 2022. PMID: 35405251 Review.
-
[Plant anti-herbivore defense priming: Concept, mechanisms and application.].Ying Yong Sheng Tai Xue Bao. 2018 Jun;29(6):2068-2078. doi: 10.13287/j.1001-9332.201806.034. Ying Yong Sheng Tai Xue Bao. 2018. PMID: 29974718 Review. Chinese.
Cited by
-
An inducible gene from glycoside hydrolase one family of Plutella xylostella decreases larval survival when feeding on host plant.Front Physiol. 2022 Oct 20;13:1013092. doi: 10.3389/fphys.2022.1013092. eCollection 2022. Front Physiol. 2022. PMID: 36338470 Free PMC article.
-
Plant sesquiterpene lactones.Philos Trans R Soc Lond B Biol Sci. 2024 Nov 18;379(1914):20230350. doi: 10.1098/rstb.2023.0350. Epub 2024 Sep 30. Philos Trans R Soc Lond B Biol Sci. 2024. PMID: 39343024 Review.
-
Harmonizing biosynthesis with post-ingestive modifications to understand the ecological functions of plant natural products.Nat Prod Rep. 2022 Jul 20;39(7):1383-1392. doi: 10.1039/d2np00019a. Nat Prod Rep. 2022. PMID: 35575224 Free PMC article. Review.
-
An integrative strategy used by the aphid Uroleucon formosanum to counter host sesquiterpene lactone defense: Insights from combined genomic and transcriptomic analysis.Insect Sci. 2025 Aug;32(4):1155-1173. doi: 10.1111/1744-7917.13452. Epub 2024 Sep 30. Insect Sci. 2025. PMID: 39350312 Free PMC article.
-
Acheta domesticus: A Natural Source of Anti-Skin-Aging Ingredients for Cosmetic Applications.Pharmaceuticals (Basel). 2024 Mar 7;17(3):346. doi: 10.3390/ph17030346. Pharmaceuticals (Basel). 2024. PMID: 38543133 Free PMC article.

