Jian-Pi-Yi-Shen formula enhances perindopril inhibition of chronic kidney disease progression by activation of SIRT3, modulation of mitochondrial dynamics, and antioxidant effects
- PMID: 34633033
- PMCID: PMC8536834
- DOI: 10.1042/BSR20211598
Jian-Pi-Yi-Shen formula enhances perindopril inhibition of chronic kidney disease progression by activation of SIRT3, modulation of mitochondrial dynamics, and antioxidant effects
Abstract
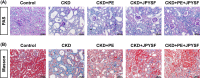
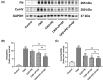
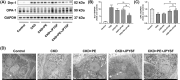
Chronic kidney disease (CKD) is a global public health problem. Renin-angiotensin system (RAS) blockade is the mainstay of CKD therapy with limitations. Jian-Pi-Yi-Shen formula (JPYSF) is a traditional herbal decoction and has been used for treating CKD for decades. The purpose of the present study was to investigate the intervention effects of combined used of perindopril erbumine (PE) and JPYSF on CKD progression and explore their underlying mechanisms. CKD rat model was induced by feeding a diet containing 0.75% w/w adenine for 3 weeks. CKD rats were treated with PE or JPYSF or PE+JPYSF from the induction of CKD and lasted 4 weeks. Renal function was evaluated by serum creatinine (Scr) and blood urea nitrogen (BUN). Pathological lesions were observed by Periodic acid-Schiff (PAS) and Masson's trichrome staining. The protein expression was tested by Western blot and immunohistochemistry analysis. The morphology of mitochondria was observed by transmission electron microscope. The results showed that combined used of PE and JPYSF could better improve renal function and pathological lesions and ameliorate renal fibrosis in CKD rats. Administration of PE and JPYSF enhanced sirtuin 3 (SIRT3) expression, inhibited mitochondrial fission, promoted mitochondrial fusion, and suppressed oxidative stress in the kidney of CKD rats. In conclusion, combined use of PE and JPYSF protected against CKD more effectively than either alone. The underlying mechanism may be associated with activation of SIRT3, modulation of mitochondrial dynamics, and antioxidant effects.
Keywords: Jian-Pi-Yi-Shen formula; chronic kidney disease; mitochondrial dynamics; oxidative stress; perindopril erbumine; sirtuins.
© 2021 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures



References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

