Post hoc analysis of a phase 3, multicenter, open-label study of cenobamate for treatment of uncontrolled focal seizures: Effects of dose adjustments of concomitant antiseizure medications
- PMID: 34633074
- PMCID: PMC9292883
- DOI: 10.1111/epi.17092
Post hoc analysis of a phase 3, multicenter, open-label study of cenobamate for treatment of uncontrolled focal seizures: Effects of dose adjustments of concomitant antiseizure medications
Abstract
Objective: To report post hoc results on how adjustments to baseline antiseizure medications (ASMs) in a subset of study sites (10 US sites) from a long-term, open-label phase 3 study of adjunctive cenobamate affected tolerability, efficacy, and retention.
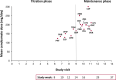
Methods: Patients with uncontrolled focal seizures taking stable doses of one to three ASMs were administered increasing doses of cenobamate (12.5, 25, 50, 100, 150, 200 mg/day) over 12 weeks at 2-week intervals (target dose = 200 mg/day). Further increases to 400 mg/day by 50 mg/day biweekly increments were allowed during maintenance phase. Dose adjustments of cenobamate and concomitant ASMs were allowed. Data were assessed until last visit, at data cut-off, on or after September 1, 2019.
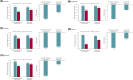
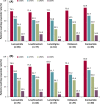
Results: A total of 240 patients meeting eligibility criteria were assessed (median [max] exposure 30.2 [43.0] months), with 177 patients continuing cenobamate at data cut-off. Most common baseline concomitant ASMs were lacosamide, levetiracetam, lamotrigine, zonisamide, and clobazam. For most baseline concomitant ASMs, ~70% of patients taking that ASM were continuing cenobamate at data cut-off. Patients continuing cenobamate had greater mean ASM dose reductions and percent dose changes from baseline vs those who discontinued. Of patients continuing cenobamate, 24.6% discontinued one or more concomitant ASMs completely. Dose decreases for all concomitant ASMs generally occurred during titration or early maintenance phases and were mostly due to central nervous system (CNS)-related adverse events such as somnolence, dizziness, unsteady gait, and fatigue. Responder rates from ≥50% through 100% for patients continuing cenobamate were generally similar regardless of concomitant ASMs (of those most commonly taken), with ~81% being ≥50% responders and ~12% achieving 100% seizure reduction in the maintenance phase, which lasted up to 40.2 (median = 29.5) months.
Significance: Concomitant ASM dose reductions were associated with more patients remaining on cenobamate. This is likely due to efficacy and improved tolerability, with overall reduced concomitant drug burden in patients with uncontrolled seizures despite taking one to three baseline concomitant ASMs.
Keywords: antiepileptics; antiseizure medications; cenobamate; concomitant medications; focal epilepsy.
© 2021 The Authors. Epilepsia published by Wiley Periodicals LLC on behalf of International League Against Epilepsy.
Conflict of interest statement
WER: Consultant/advisor: SK Life Science, Inc.; Speaker: Eisai, Greenwich Biosciences (GW Pharmaceuticals), SK Life Science, Inc., Sunovion, and UCB Pharma; and Research support: Greenwich Biosciences, Marinus, Medtronic, Neurelis, Ovid, SK Life Science, Inc., Takeda, UCB Pharma, and Upsher‐Smith.
BAK: Research support: Cerevel, Otsuka, SK Life Science, Inc., UCB Pharma, and Xenon.
SA: Consultant/advisor: Eisai, SK Life Science, Inc.; and Speaker: Eisai, Sunovion.
PB: Research support: SK Life Science, Inc.
VB: Research support: SK Life Science, Inc.
GLK: Consultant/advisor: Adamas, Eisai, Otsuka, and Shire; and Research support: Biogen, SK Life Science, Inc., UCB Pharma, and Upsher‐Smith.
MRS: Consultant/advisor: Medtronic and Neurelis; Speaker: Eisai, International Medical Press, Medscape, NeurologyLive, Projects in Knowledge, and UCB Pharma; Research support: Cavion, Cerevel, Eisai, Engage, Medtronic, Neurelis, SK Life Science, Inc., Takeda, UCB Pharma, and Xenon; and Royalty: Oxford University Press.
DGV: Consultant/advisor: Otsuka and SK Life Science, Inc.; Speaker: Greenwich Biosciences, Neurelis, SK Life Science, Inc., and UCB Pharma; and Research support: Biogen, Eisai, SK Life Science, Inc., UCB Pharma, and Xenon.
PK: Consultant/advisor: Abbott, Aquestive, Arvelle, Eisai, Engage, Neurelis, SK Life Science, Inc., and UCB Pharma; Speaker: Aquestive, Eisai, Neurelis, Sunovion, and UCB Pharma; Research support: Eisai and Lundbeck; Member, Medical Advisory Board for Alliance‐Stratus and Scientific Advisory Board for OB Pharma; and CEO, PrevEp, LLC.
RW: Consultant/advisor: Brain Sentinel, Eisai, Engage, Greenwich Biosciences, Lundbeck, SK Life Science, Inc., Sunovion, and UCB Pharma; Speaker: Aquestive, Eisai, Greenwich Biosciences, LivaNova, Sunovion, and UCB Pharma; and Research support: Aquestive, Biogen, Eisai, Engage, Greenwich Biosciences, Lundbeck, Pfizer, SK Life Science, Inc., Sunovion, UCB Pharma, Xenon, and Zogenix.
Figures
Similar articles
-
Safety and Efficacy of Cenobamate for the Treatment of Focal Seizures in Older Patients: Post Hoc Analysis of a Phase III, Multicenter, Open-Label Study.Drugs Aging. 2024 Mar;41(3):251-260. doi: 10.1007/s40266-024-01102-3. Epub 2024 Mar 6. Drugs Aging. 2024. PMID: 38446341 Free PMC article.
-
Efficacy of cenobamate for uncontrolled focal seizures: Post hoc analysis of a Phase 3, multicenter, open-label study.Epilepsia. 2021 Dec;62(12):3005-3015. doi: 10.1111/epi.17091. Epub 2021 Oct 11. Epilepsia. 2021. PMID: 34633084 Free PMC article.
-
Efficacy of adjunctive cenobamate based on number of concomitant antiseizure medications, seizure frequency, and epilepsy duration at baseline: A post-hoc analysis of a randomized clinical study.Epilepsy Res. 2021 May;172:106592. doi: 10.1016/j.eplepsyres.2021.106592. Epub 2021 Feb 18. Epilepsy Res. 2021. PMID: 33662894 Clinical Trial.
-
Indirect treatment comparison of cenobamate to other ASMs for the treatment of uncontrolled focal seizures.Epilepsy Behav. 2022 Jan;126:108429. doi: 10.1016/j.yebeh.2021.108429. Epub 2021 Dec 1. Epilepsy Behav. 2022. PMID: 34864380
-
Cenobamate: A Review in Focal-Onset Seizures.CNS Drugs. 2025 Jul;39(7):707-719. doi: 10.1007/s40263-025-01178-4. Epub 2025 Apr 14. CNS Drugs. 2025. PMID: 40227505 Review.
Cited by
-
Onset of efficacy and adverse events during Cenobamate titration period.Acta Neurol Scand. 2022 Sep;146(3):265-275. doi: 10.1111/ane.13659. Epub 2022 Jun 16. Acta Neurol Scand. 2022. PMID: 35711112 Free PMC article. Clinical Trial.
-
Safety and Efficacy of Cenobamate for the Treatment of Focal Seizures in Older Patients: Post Hoc Analysis of a Phase III, Multicenter, Open-Label Study.Drugs Aging. 2024 Mar;41(3):251-260. doi: 10.1007/s40266-024-01102-3. Epub 2024 Mar 6. Drugs Aging. 2024. PMID: 38446341 Free PMC article.
-
Seizure freedom and reducing the risk of sudden unexpected death in patients with focal epilepsy treated with cenobamate or other antiseizure medications.Epilepsia. 2025 Mar;66 Suppl 1(Suppl 1):4-14. doi: 10.1111/epi.18307. Epilepsia. 2025. PMID: 40105710 Free PMC article. Review.
-
Cenobamate in refractory epilepsy: Overview of treatment options and practical considerations.Epilepsia Open. 2023 Dec;8(4):1241-1255. doi: 10.1002/epi4.12830. Epub 2023 Oct 3. Epilepsia Open. 2023. PMID: 37743544 Free PMC article. Review.
-
Therapeutic strategies during cenobamate treatment initiation: Delphi panel recommendations.Ther Adv Neurol Disord. 2024 Jun 14;17:17562864241256733. doi: 10.1177/17562864241256733. eCollection 2024. Ther Adv Neurol Disord. 2024. PMID: 38883228 Free PMC article. Review.
References
-
- Brodie MJ, Sills GJ. Combining antiepileptic drugs–rational polytherapy? Seizure. 2011;20(5):369–75. - PubMed
-
- Dash D, Aggarwal V, Joshi R, Padma MV, Tripathi M. Effect of reduction of antiepileptic drugs in patients with drug‐refractory epilepsy. Seizure. 2015;27:25–9. - PubMed
-
- Patsalos PN, Perucca E. Clinically important drug interactions in epilepsy: interactions between antiepileptic drugs and other drugs. Lancet Neurol. 2003;2(8):473–81. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

