Efficacy of cenobamate for uncontrolled focal seizures: Post hoc analysis of a Phase 3, multicenter, open-label study
- PMID: 34633084
- PMCID: PMC9293007
- DOI: 10.1111/epi.17091
Efficacy of cenobamate for uncontrolled focal seizures: Post hoc analysis of a Phase 3, multicenter, open-label study
Abstract
Objective: To report long-term post hoc efficacy and safety data from 10 US study sites from an open-label Phase 3 study of adjunctive cenobamate (NCT02535091).
Methods: Patients with uncontrolled focal seizures taking stable doses of 1-3 antiseizure medications (ASMs) were administered increasing daily doses of cenobamate (12.5, 25, 50, 100, 150, 200 mg/day) over 12 weeks at 2-week intervals (target dose = 200 mg/day). Further increases to 400 mg/day by 50-mg/day increments biweekly were allowed during the maintenance phase. Dose adjustments of cenobamate and concomitant ASMs were allowed. Data were assessed until the last clinic visit on or after September 1, 2019.
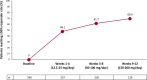
Results: Of 255 patients, 240 with focal aware motor, focal impaired awareness, or focal to bilateral tonic-clonic seizure data while on treatment were evaluated (median [maximum] exposure = 30.2 [43.0] months across the entire study). Median baseline seizure frequency/28 days was 2.8 (mean = 18.1). Of the 240 patients, 177 (73.8%) were continuing cenobamate treatment at data cutoff. The ≥50% responder rate for the total treatment duration was 71.7% (172/240). During titration, the ≥50% responder rates were 48.1% during Weeks 1-4 (12.5-25 mg/day cenobamate) and 61.7% during Weeks 5-8 (50-100 mg/day cenobamate). Among all patients who received a dose of cenobamate in the maintenance phase (n = 214), 13.1% (28/214) and 40.2% (86/214) achieved 100% and ≥90% seizure reduction during their entire maintenance treatment duration (median = 29.5 months). Among all patients, 87 (36.3%) had any consecutive ≥12-month duration of 100% seizure reduction. Common treatment-emergent adverse events among all 240 patients included fatigue (34.6%), dizziness (32.1%), and somnolence (29.6%).
Significance: This post hoc analysis of a subset of patients from the long-term open-label study showed high rates of sustained 100% and ≥90% seizure reduction, with many achieving response early during titration. These findings suggest durable seizure frequency reduction with cenobamate in adults with uncontrolled focal seizures.
Keywords: cenobamate; efficacy; focal epilepsy; long term; safety/tolerability.
© 2021 The Authors. Epilepsia published by Wiley Periodicals LLC on behalf of International League Against Epilepsy.
Conflict of interest statement
M.R.S.: Consultant/advisor: Medtronic, Neurelis; speaker: Eisai, International Medical Press, Medscape, NeurologyLive, Projects in Knowledge, UCB Pharma; research support: Cavion, Cerevel, Eisai, Engage, Medtronic, Neurelis, SK Life Science, Takeda, UCB Pharma, Xenon; royalty: Oxford University Press. B.A.‐K.: Research support: Otsuka, SK Life Science, UCB Pharma, Xenon. S.A.: Consultant/advisor: Eisai, SK Life Science; speaker: Eisai, Sunovion. P.B.: Research support: SK Life Science. V.B.: Research support: SK Life Science. P.K.: Consultant/advisor: Abbott, Aquestive, Arvelle, Eisai, Engage, Neurelis, SK Life Science, UCB Pharma; speaker: Aquestive, Eisai, Neurelis, Sunovion, UCB Pharma; research support: Eisai, Lundbeck; member, medical advisory board for Alliance‐Stratus, scientific advisory board for OB Pharma; CEO: PrevEp. G.L.K.: Consultant/advisor: Adamas, Eisai, Otsuka, Shire; research support: Biogen, SK Life Science, UCB Pharma, Upsher‐Smith. D.G.V.: Consultant/advisor: Otsuka, SK Life Science; speaker: Greenwich Biosciences, Neurelis, SK Life Science, UCB Pharma; research support: Biogen, Eisai, SK Life Science, UCB Pharma, Xenon. R.W.: Consultant/advisor: Brain Sentinel, Eisai, Engage, Greenwich Biosciences, Lundbeck, SK Life Science, Sunovion, UCB Pharma; speaker: Aquestive, Eisai, Greenwich Biosciences, LivaNova, Sunovion, UCB Pharma; research support: Aquestive, Biogen, Eisai, Engage, Greenwich Biosciences, Lundbeck, Pfizer, SK Life Science, Sunovion, UCB Pharma, Xenon, Zogenix. L.F., M.G.: Employees, SK Life Science. W.E.R.: Consultant/advisor: SK Life Science; speaker: Eisai, Greenwich Biosciences (GW Pharmaceuticals), SK Life Science, Sunovion, UCB Pharma; research support: Greenwich Biosciences, Marinus, Medtronic, Neurelis, Ovid, SK Life Science, Takeda, UCB Pharma, Upsher‐Smith. The authors confirm that they have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Figures



References
-
- Perucca P, Scheffer IE, Kiley M. The management of epilepsy in children and adults. Med J Aust. 2018;208(5):226–33. - PubMed
-
- Golyala A, Kwan P. Drug development for refractory epilepsy: the past 25 years and beyond. Seizure. 2017;44:147–56. - PubMed
-
- Nevalainen O, Ansakorpi H, Simola M, Raitanen J, Isojarvi J, Artama M, et al. Epilepsy‐related clinical characteristics and mortality: a systematic review and meta‐analysis. Neurology. 2014;83(21):1968–77. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

