A pre-screening strategy to assess resected tumor margins by imaging cytoplasmic viscosity and hypoxia
- PMID: 34633289
- PMCID: PMC8553343
- DOI: 10.7554/eLife.70471
A pre-screening strategy to assess resected tumor margins by imaging cytoplasmic viscosity and hypoxia
Abstract
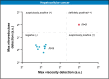
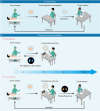
To assure complete tumor removal, frozen section analysis is the most common procedure for intraoperative pathological assessment of resected tumor margins. However, during one operation, multiple biopsies may be sent for examination, but only few of them are made into cryosections because of the complex preparation protocols and time-consuming pathological analysis, which potentially increases the risk of overlooking tumor involvement. Here, we propose a fluorescence-based pre-screening strategy that allows high-throughput, convenient, and fast gross assessment of resected tumor margins. A dual-activatable cationic fluorescent molecular rotor was developed to specifically illuminate live tumor cells' cytoplasm by emitting two different fluorescence signals in response to elevations in hypoxia-induced nitroreductase (a biochemical marker) and cytoplasmic viscosity (a biophysical marker), two characteristics of cancer cells. The ability of the fluorescent molecular rotor in detecting tumor cells was evaluated in mouse and human specimens of multiple tissues by comparing with hematoxylin and eosin staining. Importantly, the fluorescent molecular rotor achieved 100 % specificity in discriminating lung and liver cancers from normal tissue, allowing pre-screening of the tumor-free surgical margins and promoting clinical decision. Altogether, this type of fluorescent molecular rotor and the proposed strategy may serve as a new option to facilitate intraoperative assessment of resected tumor margins.
Keywords: biochemistry; chemical biology; cytoplasmic viscosity; dual-activatable; fluorescent molecular rotor; human; hypoxia; pre-screening strategy.
© 2021, Huang et al.
Conflict of interest statement
HH, YL, WM, JL, JH, XH, MT, SY, MA, CZ, QG, WZ No competing interests declared
Figures

























References
-
- Bressloff PC, Newby JM. Stochastic models of intracellular transport. Reviews of Modern Physics. 2013;85:135–196. doi: 10.1103/RevModPhys.85.135. - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

