Association Between Risk of COVID-19 Infection in Nonimmune Individuals and COVID-19 Immunity in Their Family Members
- PMID: 34633407
- PMCID: PMC8506298
- DOI: 10.1001/jamainternmed.2021.5814
Association Between Risk of COVID-19 Infection in Nonimmune Individuals and COVID-19 Immunity in Their Family Members
Abstract
Importance: The association between COVID-19 immunity within families and the risk of infection in nonimmune family members is unknown.
Objective: To investigate the association between risk of COVID-19 in nonimmune individuals and the number of their family members with known immunity acquired from a previous COVID-19 infection or full vaccination (2 vaccine doses).
Design, setting, and participants: In this cohort study of data from nationwide registries in Sweden, all individuals who acquired immunity from either previous COVID-19 infection or full vaccination until May 26, 2021, were considered for inclusion. Each person with immunity was matched 1:1 to an individual without immunity from an identified cohort of individuals with families comprising 2 to 5 members.
Exposures: Number of immune family members in each family on April 14, 2021 (index date), who acquired immunity from a previous COVID-19 infection or full vaccination (2 doses of the mRNA-1273, BNT162b2 mRNA, or ChAdOx1 nCoV-19 vaccine).
Main outcomes and measures: Incident COVID-19 infection in nonimmune family members from April 15 to May 26, 2021.
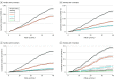
Results: A total of 1 789 728 individuals from 814 806 families were included in the analysis. Each family comprised 2 to 5 family members, with a mean (SD) age at baseline of 51.3 (19.5) years. During a mean (range) follow-up time of 26.3 (1-40) days, 88 797 of 1 549 989 (5.7%) nonimmune family members (mean [SD] age, 51.6 [17.7] years; 790 276 men [51.0%]) were diagnosed with COVID-19. There was an inverse dose-response association between the number of immune members in each family and the risk of incident COVID-19 infection in nonimmune family members. Nonimmune families with 1 immune family member had a 45% to 61% lower risk of contracting COVID-19 (hazard ratio [HR], 0.39-0.55; 95% CI, 0.37-0.61, P < .001). The risk reduction increased to 75% to 86% in families with 2 immune family members (HR, 0.14-0.25; 95% CI, 0.11-0.27; P < .001), 91% to 94% with 3 immune family members (HR, 0.06-0.09; 95% CI, 0.04-0.10; P < .001), and 97% with 4 immune family members (HR, 0.03; 95% CI, 0.02-0.05; P < .001). The results were similar for the outcome of COVID-19 infection that was severe enough to warrant a hospital stay.
Conclusions and relevance: In this cohort study, family members without immunity had a 45% to 97% lower risk of contracting COVID-19 as the number of immune family members increased. Vaccination is a key strategy for decreasing the transmission of the virus within families.
Conflict of interest statement
Figures

References
-
- European Centre for Disease Prevention and Control . COVID-19 situation update worldwide, as of week 19, updated 20 May 2021. Accessed June 14, 2021. https://www.ecdc.europa.eu/en/geographical-distribution-2019-ncov-cases
-
- World Health Organization . WHO coronavirus (COVID-19) dashboard. Accessed June 14, 2021. https://covid19.who.int
-
- Voysey M, Clemens SAC, Madhi SA, et al. ; Oxford COVID Vaccine Trial Group . Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99-111. doi:10.1016/S0140-6736(20)32661-1 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

