Comparison of New Pharmacologic Agents With Triptans for Treatment of Migraine: A Systematic Review and Meta-analysis
- PMID: 34633423
- PMCID: PMC8506232
- DOI: 10.1001/jamanetworkopen.2021.28544
Comparison of New Pharmacologic Agents With Triptans for Treatment of Migraine: A Systematic Review and Meta-analysis
Abstract
Importance: New therapeutic classes of migraine-specific treatment have been developed, including 5-hydroxytryptamine1F receptor agonists (lasmiditan) and calcitonin gene-related peptide antagonists (rimegepant and ubrogepant).
Objective: To compare outcomes associated with the use of lasmiditan, rimegepant, and ubrogepant vs triptans for acute management of migraine headaches.
Data sources: The Cochrane Register of Controlled Trials, Embase, and PubMed were searched from inception to March 5, 2020.
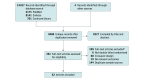
Study selection: Double-blind randomized clinical trials examining current available migraine-specific acute treatments were included.
Data extraction and synthesis: The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline was applied to extract the data according to a predetermined list of variables of interest, and all network meta-analyses were conducted using a random-effects model.
Main outcomes and measures: The primary outcome was the odds ratio (OR) for freedom from pain (hereafter referred to as pain freedom) at 2 hours after the dose, and the secondary outcomes were ORs for pain relief at 2 hours after the dose and any adverse events.
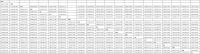
Results: A total of 64 randomized clinical trials were included (46 442 participants; 74%-87% women; age range, 36-43 years). Most of the included treatments were associated with reduced pain at 2 hours compared with placebo. Most triptans were associated with higher ORs for pain freedom at 2 hours compared with lasmiditan (range: OR, 1.72 [95% CI, 1.06-2.80] to OR, 3.40 [95% CI, 2.12-5.44]), rimegepant (range: OR, 1.58 [95% CI, 1.07-2.33] to OR, 3.13 [95% CI, 2.16-4.52]), and ubrogepant (range: OR, 1.54 [95% CI, 1.00-2.37] to OR, 3.05 [95% CI, 2.02-4.60]). Most triptans were associated with higher ORs for pain relief at 2 hours compared with lasmiditan (range: OR, 1.46 [95% CI, 1.09-1.96] to OR, 3.31 [95% CI, 2.41-4.55]), rimegepant (range: OR, 1.33 [95% CI, 1.01-1.76] to OR, 3.01 [95% CI, 2.33-3.88]), and ubrogepant (range: OR, 1.38 [95% CI, 1.02-1.88] to OR, 3.13 [95% CI, 2.35-4.15]). The comparisons between lasmiditan, rimegepant, and ubrogepant were not statistically significant for both pain freedom and pain relief at 2 hours. Lasmiditan was associated with the highest risk of any adverse events, and certain triptans (rizatriptan, sumatriptan, and zolmitriptan) were also associated with a higher risk of any adverse events than the calcitonin gene-related peptide antagonists.
Conclusions and relevance: For pain freedom or pain relief at 2 hours after the dose, lasmiditan, rimegepant, and ubrogepant were associated with higher ORs compared with placebo but lower ORs compared with most triptans. However, the lack of cardiovascular risks for these new classes of migraine-specific treatments may offer an alternative to triptans.
Conflict of interest statement
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

