Regulation of innate immune responses in macrophages differentiated in the presence of vitamin D and infected with dengue virus 2
- PMID: 34634046
- PMCID: PMC8530315
- DOI: 10.1371/journal.pntd.0009873
Regulation of innate immune responses in macrophages differentiated in the presence of vitamin D and infected with dengue virus 2
Abstract
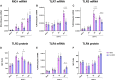
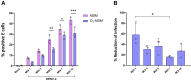
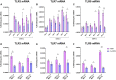
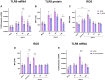
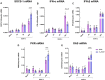
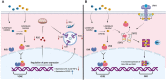
A dysregulated or exacerbated inflammatory response is thought to be the key driver of the pathogenesis of severe disease caused by the mosquito-borne dengue virus (DENV). Compounds that restrict virus replication and modulate the inflammatory response could thus serve as promising therapeutics mitigating the disease pathogenesis. We and others have previously shown that macrophages, which are important cellular targets for DENV replication, differentiated in the presence of bioactive vitamin D (VitD3) are less permissive to viral replication, and produce lower levels of pro-inflammatory cytokines. Therefore, we here evaluated the extent and kinetics of innate immune responses of DENV-2 infected monocytes differentiated into macrophages in the presence (D3-MDMs) or absence of VitD3 (MDMs). We found that D3-MDMs expressed lower levels of RIG I, Toll-like receptor (TLR)3, and TLR7, as well as higher levels of SOCS-1 in response to DENV-2 infection. D3-MDMs produced lower levels of reactive oxygen species, related to a lower expression of TLR9. Moreover, although VitD3 treatment did not modulate either the expression of IFN-α or IFN-β, higher expression of protein kinase R (PKR) and 2'-5'-oligoadenylate synthetase 1 (OAS1) mRNA were found in D3-MDMs. Importantly, the observed effects were independent of reduced infection, highlighting the intrinsic differences between D3-MDMs and MDMs. Taken together, our results suggest that differentiation of MDMs in the presence of VitD3 modulates innate immunity in responses to DENV-2 infection.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

