A hybridoma-derived monoclonal antibody with high homology to the aberrant myeloma light chain
- PMID: 34634047
- PMCID: PMC8504763
- DOI: 10.1371/journal.pone.0252558
A hybridoma-derived monoclonal antibody with high homology to the aberrant myeloma light chain
Abstract
The identification of antibody variable regions in the heavy (VH) and light (VL) chains from hybridomas is necessary for the production of recombinant, sequence-defined monoclonal antibodies (mAbs) and antibody derivatives. This process has received renewed attention in light of recent reports of hybridomas having unintended specificities due to the production of non-antigen specific heavy and/or light chains for the intended antigen. Here we report a surprising finding and potential pitfall in variable domain sequencing of an anti-human CD63 hybridoma. We amplified multiple VL genes from the hybridoma cDNA, including the well-known aberrant Sp2/0 myeloma VK and a unique, full-length VL. After finding that the unique VL failed to yield a functional antibody, we discovered an additional full-length sequence with surprising similarity (~95% sequence identify) to the non-translated myeloma kappa chain but with a correction of its key frameshift mutation. Expression of the recombinant mAb confirmed that this highly homologous sequence is the antigen-specific light chain. Our results highlight the complexity of PCR-based cloning of antibody genes and strategies useful for identification of correct sequences.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

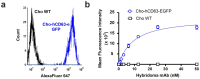
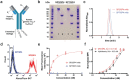
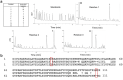
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

