Non-clinical assessment of lubrication and free radical scavenging of an innovative non-animal carboxymethyl chitosan biomaterial for viscosupplementation: An in-vitro and ex-vivo study
- PMID: 34634053
- PMCID: PMC8504732
- DOI: 10.1371/journal.pone.0256770
Non-clinical assessment of lubrication and free radical scavenging of an innovative non-animal carboxymethyl chitosan biomaterial for viscosupplementation: An in-vitro and ex-vivo study
Abstract
Objective: Lubrication and free radical scavenging are key features of biomaterials used for viscosupplementation (VS) of joints affected by osteoarthritis (OA). The objective of this study was to describe the non-clinical performance characterization of KiOmedine® CM-Chitosan, a non-animal carboxymethyl chitosan, in order to assess its intended action in VS and to compare it to existing viscosupplements based on crosslinked hyaluronan (HA) formulations.
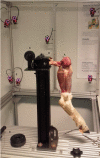
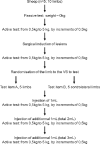
Method: The lubrication capacity of the tested viscosupplements (VS) was evaluated in-vitro and ex-vivo. In-vitro, the coefficient of friction (COF) was measured using a novel tribological system. Meanwhile, an ex-vivo biomechanical model in ovine hindlimbs was developed to assess the recovery of join mobility after an intra-articular (IA) injection. Free radical scavenging capacity of HA and KiOmedine® CM-Chitosan formulations was evaluated using the Trolox Equivalent Antioxidant Capacity (TEAC) assay.
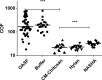
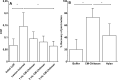
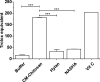
Results: In the in-vitro tribological model, KiOmedine® CM-Chitosan showed high lubrication capacity with a significant COF reduction than crosslinked HA formulations. In the ex-vivo model, the lubrication effect of KiOmedine® CM-Chitosan following an IA injection in the injured knee was proven again by a COF reduction. The recovery of joint motion was optimal with an IA injection of 3 ml of KiOmedine® CM-Chitosan, which was significantly better than the crosslinked HA formulation at the same volume. In the in-vitro TEAC assay, KiOmedine® CM-Chitosan showed a significantly higher free radical scavenging capacity than HA formulations.
Conclusion: Overall, the results provide a first insight into the mechanism of action in terms of lubrication and free radical scavenging for the use of KiOmedine® CM-Chitosan as a VS treatment of OA. KiOmedine® CM-Chitosan demonstrated a higher capacity to scavenge free radicals, and it showed a higher recovery of mobility after a knee lesion than crosslinked HA formulations. This difference could be explained by the difference in chemical structure between KiOmedine® CM-Chitosan and HA and their formulations.
Conflict of interest statement
The sheep study and biomechanical characterization (at Namur university and Université Libre de Bruxelles) were sponsored by KiOmed Pharma, Herstal, Belgium. Jean-Michel Vandeweerd and Fanny Hontoir are paid consultants for KiOmed Pharma. This did not affect the way in which the results of this paper were analysed and reported. Bernardo Innocenti reports no conflicts of interest that could impact the research. Guillem Rocasalbas, Sandrine Gautier and Mickaël Chausson are full-time employees of KiOmed Pharma. Laurence Hermitte is a full-time consultant for KiOmed Pharma. Pierre Douette was, at the time of the study, a full-time consultant for KiOmed Pharma. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures


F = quadriceps force (in the experimental analysis obtained as the force of the weight);
Fx = component of the force F along the direction X
Fy = component of the force F along the direction Y
m = mass of the limb;
mg = weight of the limb (g is the gravity, 9.81 m/s2);
F* = the contact force between the tibia and the femur;
f = friction coefficient (or COF);
fF* = the friction force;
r = radius of the femoral condyle;
a = distance between the tibial insertion point of the patellar tendon and the hinge centre;
b = distance between the barycentre of the lower leg and the hinge centre;
α (alpha) = flexion angle;
= the second derivative (angular acceleration) of α with respect to the time
I0 = the mass moment of inertia
β (beta) = angle from the patellar tendon and the tibial axis
References
-
- Felson D.T. NIH Conference Osteoarthritis: New Insights. Ann Intern Med [Internet]. 2000;133(8):637–9.
-
- Healy Z.R., Lee N.H., Gao X., Goldring M.B., Talalay P., Kensler T.W., et al. Divergent responses of chondrocytes and endothelial cells to shear stress: Cross-talk among COX-2, the phase 2 response, and apoptosis. Proc Natl Acad Sci U S A. 2005;102(39):14010–5. doi: 10.1073/pnas.0506620102 - DOI - PMC - PubMed
-
- Pelletier J.P., Boileau C., Altman R.D., Martel-Pelletier J. Experimental models of osteoarthritis: Usefulness in the development of disease-modifying osteoarthritis drugs/agents. Therapy. 2010;7(6):621–34.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

