Systemic inflammatory response in robot-assisted and laparoscopic surgery for colon cancer (SIRIRALS): study protocol of a randomized controlled trial
- PMID: 34635066
- PMCID: PMC8507379
- DOI: 10.1186/s12893-021-01355-4
Systemic inflammatory response in robot-assisted and laparoscopic surgery for colon cancer (SIRIRALS): study protocol of a randomized controlled trial
Abstract
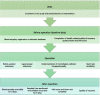
Background: Robot-assisted surgery is being increasingly adopted in treating colorectal cancer, and the transition from laparoscopic surgery to robot-assisted surgery is a trend. The evidence of the benefits of robot-assisted surgery is sparse. However, findings are associated with improved patient-related outcomes and overall morbidity rates compared to laparoscopic surgery. This induction is unclear, considering both surgical modalities are characterized as minimally invasive. This study aims to evaluate the systemic and peritoneal inflammatory stress response induced by robot-assisted surgery compared with laparoscopic surgery for elective colon cancer resections in a prospective, randomized controlled clinical trial.
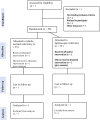
Methods: This study is a single-centre randomized controlled superiority trial with 50 colon cancer participants. The primary endpoint is the level of systemic inflammatory response expressed as serum C-reactive protein (CRP) and interleukin 6 (IL-6) levels between postoperative days one and three. Secondary endpoints include (i) levels of systemic inflammation in serum expressed by a panel of inflammatory and pro-inflammatory cytokines measured during the first three postoperative days, (ii) postoperative surgical and medical complications (30 days) according to Clavien-Dindo classification and Comprehensive Complication Index, (iii) intraoperative blood loss, (iv) conversion rate to open surgery, (v) length of surgery, (vi) operative time, (vii) the number of harvested lymph nodes, and (viii) length of hospital stay. The exploratory endpoints are (i) levels of peritoneal inflammatory response in peritoneal fluid expressed by inflammatory and pro-inflammatory cytokines between postoperative day one and three, (ii) patient-reported health-related quality of recovery-15 (QoR-15), (iii) 30 days mortality rate, (iv) heart rate variability and (v) gene transcript (mRNA) analysis.
Discussion: To our knowledge, this is the first clinical randomized controlled trial to clarify the inflammatory stress response induced by robot-assisted or laparoscopic surgery for colon cancer resections. Trial registration This trial is registered at Clinicaltrials.gov (Identifier: NCT04687384) on December, 29, 2020, Regional committee on health research ethics, Region of Southern Denmark (N75709) and Data Protection Agency, Hospital Sønderjylland, University Hospital of Southern Denmark (N20/46179).
Keywords: Colon cancer; Inflammatory surgical stress response; Laparoscopic surgery; Minimally invasive surgery; Robot-assisted surgery.
© 2021. The Author(s).
Conflict of interest statement
The main investigator and collaborators have no financial interest in the trial.
Figures
Similar articles
-
Surgical stress response in robot-assisted versus laparoscopic surgery for colon cancer (SIRIRALS): randomized clinical trial.Br J Surg. 2024 Mar 2;111(3):znae049. doi: 10.1093/bjs/znae049. Br J Surg. 2024. PMID: 38445434 Clinical Trial.
-
Improved perioperative outcomes and reduced inflammatory stress response in malignant robot-assisted colorectal resections: a retrospective cohort study of 298 patients.World J Surg Oncol. 2021 May 22;19(1):155. doi: 10.1186/s12957-021-02263-w. World J Surg Oncol. 2021. PMID: 34022914 Free PMC article.
-
A novel hand-assisted laparoscopic versus conventional laparoscopic right hemicolectomy for right colon cancer: study protocol for a randomized controlled trial.Trials. 2017 Jul 26;18(1):355. doi: 10.1186/s13063-017-2084-3. Trials. 2017. PMID: 28747220 Free PMC article. Clinical Trial.
-
Robot-assisted surgery with Senhance robotic system for colon cancer: our original single-incision plus 2-port procedure and a review of the literature.Tech Coloproctol. 2021 Apr;25(4):467-471. doi: 10.1007/s10151-020-02389-1. Epub 2021 Feb 15. Tech Coloproctol. 2021. PMID: 33587212 Review.
-
Robot-assisted versus laparoscopic-assisted surgery for colorectal cancer: a meta-analysis.Surg Endosc. 2016 Dec;30(12):5601-5614. doi: 10.1007/s00464-016-4892-z. Epub 2016 Jul 11. Surg Endosc. 2016. PMID: 27402096 Review.
Cited by
-
The value of systemic inflammatory response index in evaluating the prognosis of postoperative patients with stage II colon cancer.BMC Gastroenterol. 2025 Aug 13;25(1):581. doi: 10.1186/s12876-025-04186-2. BMC Gastroenterol. 2025. PMID: 40804370 Free PMC article.
-
Robotic-Assisted versus Laparoscopic Left Hemicolectomy-Postoperative Inflammation Status, Short-Term Outcome and Cost Effectiveness.Int J Environ Res Public Health. 2022 Aug 25;19(17):10606. doi: 10.3390/ijerph191710606. Int J Environ Res Public Health. 2022. PMID: 36078317 Free PMC article.
-
Role of vitamin D supplementation in modifying outcomes after surgery: a systematic review of randomised controlled trials.BMJ Open. 2024 Jan 17;14(1):e073431. doi: 10.1136/bmjopen-2023-073431. BMJ Open. 2024. PMID: 38233048 Free PMC article.
-
Robotic-assisted surgery for left-sided colon and rectal resections is associated with reduction in the postoperative surgical stress response and improved short-term outcomes: a cohort study.Surg Endosc. 2024 May;38(5):2577-2592. doi: 10.1007/s00464-024-10749-3. Epub 2024 Mar 18. Surg Endosc. 2024. PMID: 38498212 Free PMC article.
References
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous