Zinc-mediated activation of CREB pathway in proliferation of pulmonary artery smooth muscle cells in pulmonary hypertension
- PMID: 34635097
- PMCID: PMC8504081
- DOI: 10.1186/s12964-021-00779-y
Zinc-mediated activation of CREB pathway in proliferation of pulmonary artery smooth muscle cells in pulmonary hypertension
Abstract
Background: Transcription factor CREB is involved in the development of pulmonary hypertension (PH). However, little is known about the role and regulatory signaling of CREB in PH.
Methods: A series of techniques, including bioinformatics methods, western blot, cell proliferation and luciferase reporter assay were used to perform a comprehensive analysis of the role and regulation of CREB in proliferation of pulmonary artery smooth muscle cells (PASMCs) in PH.
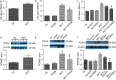
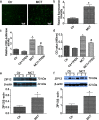
Results: Using bioinformatic analysis of the differentially expressed genes (DEGs) identified in the development of monocrotaline (MCT)- and hypoxia-induced PH, we found the overrepresentation of CRE-containing DEGs. Western blot analysis revealed a sustained increase in total- and phosphorylated-CREB in PASMCs isolated from rats treated with MCT. Similarly, an enhanced and prolonged serum-induced CREB phosphorylation was observed in hypoxia-pretreated PASMCs. The sustained CREB phosphorylation in PASMCs may be associated with multiple protein kinases phosphorylated CREB. Additionally, hierarchical clustering analysis showed reduced expression of the majority of CREB phosphatases in PH, including regulatory subunits of PP2A, Ppp2r2c and Ppp2r3a. Cell proliferation analysis showed increased PASMCs proliferation in MCT-induced PH, an effect relied on CREB-mediated transcriptional activity. Further analysis revealed the raised intracellular labile zinc possibly from ZIP12 was associated with reduced phosphatases, increased CREB-mediated transcriptional activity and PASMCs proliferation.
Conclusions: CREB pathway was overactivated in the development of PH and contributed to PASMCs proliferation, which was associated with multiple protein kinases and/or reduced CREB phosphatases and raised intracellular zinc. Thus, this study may provide a novel insight into the CREB pathway in the pathogenesis of PH. Video abstract.
Keywords: CREB; Intracellular labile zinc; Protein phosphatases; Pulmonary artery smooth muscle cells proliferation; Pulmonary hypertension.
Plain language summary
Transcription factor CREB plays an important role in the development of pulmonary hypertension (PH). However, paradoxical roles have been reported in the pathogenesis of PH, and the regulatory mechanisms of CREB activation in pulmonary artery smooth muscle cells (PASMCs) proliferation remained unknown. In this study, we showed that CRE-containing genes were overrepresented among the differentially expressed genes in experimental PH, which resulted from the sustained activation of CREB pathway. The sustained activation of CREB pathway may be associated with the activation of multiple protein kinases that positively regulate CREB and down-regulation of numerous phosphatases involved in CREB dephosphorylation. Additionally, we found that the proliferation of PAMSCs was dependent on the CREB-mediated transcriptional activity in experimental PH. Moreover, the raised intracellular labile zinc possibly from ZIP12 may be associated with reduced protein phosphatases, increased CREB-mediated transcriptional activity and PASMCs proliferation. Collectively, we found CREB-mediated transcriptional activity in the proliferation of PASMCs in PH, which may be associated with multiple protein kinases and/or reduced phosphatases and elevated intracellular zinc. This study may reveal a critical role of zinc-mediated activation of CREB pathway in the proliferation of PASMCs, thus providing a more comprehensive understanding of CREB pathway in the pathogenesis of PH.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





Similar articles
-
The zinc transporter ZIP12 regulates monocrotaline-induced proliferation and migration of pulmonary arterial smooth muscle cells via the AKT/ERK signaling pathways.BMC Pulm Med. 2022 Mar 28;22(1):111. doi: 10.1186/s12890-022-01905-3. BMC Pulm Med. 2022. PMID: 35346134 Free PMC article.
-
Aloperine protects pulmonary hypertension via triggering PPARγ signaling and inhibiting calcium regulatory pathway in pulmonary arterial smooth muscle cells.Am J Physiol Cell Physiol. 2023 Oct 1;325(4):C1058-C1072. doi: 10.1152/ajpcell.00286.2023. Epub 2023 Sep 4. Am J Physiol Cell Physiol. 2023. PMID: 37661916
-
β-sitosterol alleviates pulmonary arterial hypertension by altering smooth muscle cell phenotype and DNA damage/cGAS/STING signaling.Phytomedicine. 2024 Dec;135:156030. doi: 10.1016/j.phymed.2024.156030. Epub 2024 Sep 7. Phytomedicine. 2024. PMID: 39265206
-
Implications of Hydrogen Sulfide in Development of Pulmonary Hypertension.Biomolecules. 2022 Jun 1;12(6):772. doi: 10.3390/biom12060772. Biomolecules. 2022. PMID: 35740897 Free PMC article. Review.
-
MicroRNAs in Pulmonary Hypertension, from Pathogenesis to Diagnosis and Treatment.Biomolecules. 2022 Mar 24;12(4):496. doi: 10.3390/biom12040496. Biomolecules. 2022. PMID: 35454085 Free PMC article. Review.
Cited by
-
Bioinformatics analysis of the immune cell infiltration characteristics and correlation with crucial diagnostic markers in pulmonary arterial hypertension.BMC Pulm Med. 2023 Aug 15;23(1):300. doi: 10.1186/s12890-023-02584-4. BMC Pulm Med. 2023. PMID: 37582718 Free PMC article.
-
Zinc promotes cell proliferation via regulating metal-regulatory transcription factor 1 expression and transcriptional activity in pulmonary arterial hypertension.Cell Cycle. 2023 May;22(10):1284-1301. doi: 10.1080/15384101.2023.2205209. Epub 2023 May 1. Cell Cycle. 2023. PMID: 37128643 Free PMC article.
-
IGFBP7 promotes endothelial cell repair in the recovery phase of acute lung injury.Clin Sci (Lond). 2024 Jul 3;138(13):797-815. doi: 10.1042/CS20240179. Clin Sci (Lond). 2024. PMID: 38840498 Free PMC article.
-
Hypoxia-induced histone lactylation promotes pulmonary arterial smooth muscle cells proliferation in pulmonary hypertension.Mol Cell Biochem. 2025 Jun 30. doi: 10.1007/s11010-025-05342-8. Online ahead of print. Mol Cell Biochem. 2025. PMID: 40588665
-
The zinc transporter ZIP12 regulates monocrotaline-induced proliferation and migration of pulmonary arterial smooth muscle cells via the AKT/ERK signaling pathways.BMC Pulm Med. 2022 Mar 28;22(1):111. doi: 10.1186/s12890-022-01905-3. BMC Pulm Med. 2022. PMID: 35346134 Free PMC article.
References
-
- Humbert M, Morrell NW, Archer SL, Stenmark KR, MacLean MR, Lang IM, Christman BW, Weir EK, Eickelberg O, Voelkel NF, Rabinovitch M: Cellular and molecular pathobiology of pulmonary arterial hypertension. J Am Coll Cardiol. 2004; 43(12 Suppl S):13s–24s. - PubMed
-
- Tokunou T, Shibata R, Kai H, Ichiki T, Morisaki T, Fukuyama K, Ono H, Iino N, Masuda S, Shimokawa H, et al. Apoptosis induced by inhibition of cyclic AMP response element-binding protein in vascular smooth muscle cells. Circulation. 2003;108(10):1246–1252. doi: 10.1161/01.CIR.0000085164.13439.89. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

