SARS-CoV-2 detection with aptamer-functionalized gold nanoparticles
- PMID: 34635231
- PMCID: PMC8409056
- DOI: 10.1016/j.talanta.2021.122841
SARS-CoV-2 detection with aptamer-functionalized gold nanoparticles
Abstract
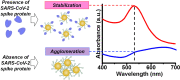
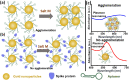
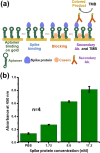
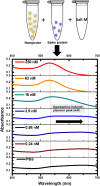
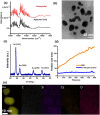
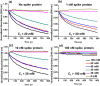
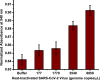
A rapid detection test for SARS-CoV-2 is urgently required to monitor virus spread and containment. Here, we describe a test that uses nanoprobes, which are gold nanoparticles functionalized with an aptamer specific to the spike membrane protein of SARS-CoV-2. An enzyme-linked immunosorbent assay confirms aptamer binding with the spike protein on gold surfaces. Protein recognition occurs by adding a coagulant, where nanoprobes with no bound protein agglomerate while those with sufficient bound protein do not. Using plasmon absorbance spectra, the nanoprobes detect 16 nM and higher concentrations of spike protein in phosphate-buffered saline. The time-varying light absorbance is examined at 540 nm to determine the critical coagulant concentration required to agglomerates the nanoprobes, which depends on the protein concentration. This approach detects 3540 genome copies/μl of inactivated SARS-CoV-2.
Keywords: Aptamer; Biosensing; Gold nanoparticle; SARS-CoV-2; Spike protein; Surface plasmon resonance.
Copyright © 2021 Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
References
-
- Rabiee N., Bagherzadeh M., Ghasemi A., Zare H., Ahmadi S., Fatahi Y., Dinarvand R., Rabiee M., Ramakrishna S., Shokouhimehr M., Varma R.S. Point-of-Use rapid detection of SARS-CoV-2: nanotechnology-enabled solutions for the COVID-19 pandemic. Int. J. Mol. Sci. 2020;21:5126. doi: 10.3390/ijms21145126. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

