Orexin-1 receptor signaling in ventral tegmental area mediates cue-driven demand for cocaine
- PMID: 34635803
- PMCID: PMC8782853
- DOI: 10.1038/s41386-021-01173-5
Orexin-1 receptor signaling in ventral tegmental area mediates cue-driven demand for cocaine
Abstract
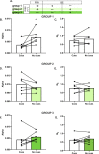
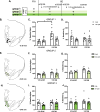
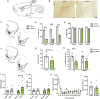
Drug-associated sensory cues increase motivation for drug and the orexin system is importantly involved in this stimulus-enhanced motivation. Ventral tegmental area (VTA) is a major target by which orexin signaling modulates reward behaviors, but it is unknown whether this circuit is necessary for cue-driven motivation for cocaine. Here, we investigated the role of VTA orexin signaling in cue-driven motivation for cocaine using a behavioral economics (BE) paradigm. We found that infusion of the orexin-1 receptor (Ox1R) antagonist SB-334867 (SB) into VTA prior to BE testing reduced motivation when animals were trained to self-administer cocaine with discrete cues and tested on BE with those cues. SB had no effect when animals were trained to self-administer cocaine without cues or tested on BE without cues, indicating that learning to associate cues with drug delivery during self-administration training was necessary for cues to recruit orexin signaling in VTA. These effects were specific to VTA, as injections of SB immediately dorsal had no effect. Moreover, intra-VTA SB did not have an impact on locomotor activity, or low- or high-effort consumption of sucrose. Finally, we microinjected a novel retrograde adeno-associated virus (AAVretro) containing an orexin-specific short hairpin RNA (OxshRNA) into VTA to knock down orexin in the hypothalamus-VTA circuit. These injections significantly reduced orexin expression in lateral hypothalamus (LH) and decreased cue-driven motivation. These studies demonstrate a role for orexin signaling in VTA, specifically when cues predict drug reward.
© 2021. The Author(s), under exclusive licence to American College of Neuropsychopharmacology.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

