Delta variant (B.1.617.2) sublineages do not show increased neutralization resistance
- PMID: 34635807
- PMCID: PMC8503871
- DOI: 10.1038/s41423-021-00772-y
Delta variant (B.1.617.2) sublineages do not show increased neutralization resistance
Conflict of interest statement
The authors declare no competing interests.
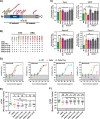
Figures
References
-
- Bosnjak B, Stein SC, Willenzon S, Cordes AK, Puppe W, Bernhardt G, et al. Low serum neutralizing anti-SARS-CoV-2 S antibody levels in mildly affected COVID-19 convalescent patients revealed by two different detection methods. Cell Mol Immunol. 2021;18:936–44. doi: 10.1038/s41423-020-00573-9. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 01KI2006D, 01KI20328A, 01KI20396, 01KX2021/Bundesministerium für Bildung und Forschung (Federal Ministry of Education and Research)
- 01KI2043, 01KX2021/Bundesministerium für Bildung und Forschung (Federal Ministry of Education and Research)
- PO 716/11-1, PO 716/14-1/Deutsche Forschungsgemeinschaft (German Research Foundation)
- RTG1660, TRR130/Deutsche Forschungsgemeinschaft (German Research Foundation)
- 14-76103-184, MWK HZI COVID-19/Niedersächsische Ministerium für Wissenschaft und Kultur (Lower Saxony Ministry of Science and Culture)
LinkOut - more resources
Full Text Sources
Medical

