Renal plasticity revealed through reversal of polycystic kidney disease in mice
- PMID: 34635846
- PMCID: PMC9278957
- DOI: 10.1038/s41588-021-00946-4
Renal plasticity revealed through reversal of polycystic kidney disease in mice
Abstract
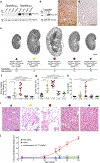
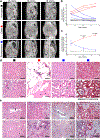
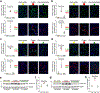
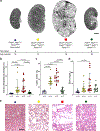
Initiation of cyst formation in autosomal dominant polycystic kidney disease (ADPKD) occurs when kidney tubule cells are rendered null for either PKD1 or PKD2 by somatic 'second hit' mutations. Subsequent cyst progression remodels the organ through changes in tubule cell shape, proliferation and secretion. The kidney develops inflammation and fibrosis. We constructed a mouse model in which adult inactivation of either Pkd gene can be followed by reactivation of the gene at a later time. Using this model, we show that re-expression of Pkd genes in cystic kidneys results in rapid reversal of ADPKD. Cyst cell proliferation is reduced, autophagy is activated and cystic tubules with expanded lumina lined by squamoid cells revert to normal lumina lined by cuboidal cells. Increases in inflammation, extracellular matrix deposition and myofibroblast activation are reversed, and the kidneys become smaller. We conclude that phenotypic features of ADPKD are reversible and that the kidney has an unexpected capacity for plasticity controlled at least in part by ADPKD gene function.
© 2021. The Author(s), under exclusive licence to Springer Nature America, Inc.
Conflict of interest statement
Competing Interests
The authors declare no competing interests.
Figures




Comment in
-
Reversal of PKD in mice.Nat Rev Nephrol. 2021 Dec;17(12):794. doi: 10.1038/s41581-021-00509-0. Nat Rev Nephrol. 2021. PMID: 34697488 No abstract available.
-
Reversing polycystic kidney disease.Nat Genet. 2021 Dec;53(12):1623-1624. doi: 10.1038/s41588-021-00963-3. Nat Genet. 2021. PMID: 34737430 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

