Comparative clinical effectiveness and safety of tobacco cessation pharmacotherapies and electronic cigarettes: a systematic review and network meta-analysis of randomized controlled trials
- PMID: 34636108
- PMCID: PMC9293179
- DOI: 10.1111/add.15675
Comparative clinical effectiveness and safety of tobacco cessation pharmacotherapies and electronic cigarettes: a systematic review and network meta-analysis of randomized controlled trials
Abstract
Aim: To determine how varenicline, bupropion, nicotine replacement therapy (NRT) and electronic cigarettes compare with respect to their clinical effectiveness and safety.
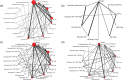
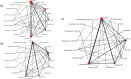
Method: Systematic reviews and Bayesian network meta-analyses of randomized controlled trials, in any setting, of varenicline, bupropion, NRT and e-cigarettes (in high, standard and low doses, alone or in combination) in adult smokers and smokeless tobacco users with follow-up duration of 24 weeks or greater (effectiveness) or any duration (safety). Nine databases were searched until 19 February 2019. Primary outcomes were sustained tobacco abstinence and serious adverse events (SAEs). We estimated odds ratios (ORs) and treatment rankings and conducted meta-regression to explore covariates.
Results: We identified 363 trials for effectiveness and 355 for safety. Most monotherapies and combination therapies were more effective than placebo at helping participants to achieve sustained abstinence; the most effective of these, estimated with some imprecision, were varenicline standard [OR = 2.83, 95% credible interval (CrI) = 2.34-3.39] and varenicline standard + NRT standard (OR = 5.75, 95% CrI = 2.27-14.88). Estimates were higher in smokers receiving counselling than in those without and in studies with higher baseline nicotine dependence scores than in those with lower scores. Varenicline standard + NRT standard showed a high probability of being ranked best or second-best. For safety, only bupropion at standard dose increased the odds of experiencing SAEs compared with placebo (OR = 1.27, 95% CrI = 1.04-1.58), and we found no evidence of effect modification.
Conclusions: Most tobacco cessation monotherapies and combination therapies are more effective than placebo at helping participants to achieve sustained abstinence, with varenicline appearing to be most effective based on current evidence. There does not appear to be strong evidence of associations between most tobacco cessation pharmacotherapies and adverse events; however, the data are limited and there is a need for improved reporting of safety data.
Keywords: adverse events; bupropion; effectiveness; electronic cigarettes; network meta-analysis; nicotine replacement therapy; safety; smoking; varenicline.
© 2021 The Authors. Addiction published by John Wiley & Sons Ltd on behalf of Society for the Study of Addiction.
Figures



References
-
- Jha P, Peto R. Global effects of smoking, of quitting, and of taxing tobacco. N Engl J Med. 2014;370:60–8. - PubMed
-
- National Institute for Health and Clinical Excellence (NICE) . NICE Guideline [NG92] Stop Smoking Interventions and Services. London: NICE; 2018.
-
- McNeill ABL, Calder R, Hitchman SC, Hajek P, McRobbie H. E‐Cigarettes: An Evidence Update—A Report Commissioned by Public Health England. London: Public Health England; 2015.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

