The safety of activated eptacog beta in the management of bleeding episodes and perioperative haemostasis in adult and paediatric haemophilia patients with inhibitors
- PMID: 34636112
- PMCID: PMC9292935
- DOI: 10.1111/hae.14419
The safety of activated eptacog beta in the management of bleeding episodes and perioperative haemostasis in adult and paediatric haemophilia patients with inhibitors
Abstract
Introduction: Haemophilia patients with inhibitors often require a bypassing agent (BPA) for bleeding episode management. Eptacog beta (EB) is a new FDA-approved recombinant activated human factor VII BPA for the treatment and control of bleeding in haemophilia A or B patients with inhibitors (≥12 years of age). We describe here the EB safety profile from the three prospective Phase 3 clinical trials performed to date.
Aim: To assess EB safety, immunogenicity and thrombotic potential in children and adults who received EB for treatment of bleeding and perioperative care.
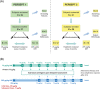
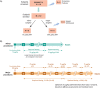
Methods: Using a randomized crossover design, 27 subjects in PERSEPT 1 (12-54 years) and 25 subjects in PERSEPT 2 (1-11 years) treated bleeding episodes with 75 or 225 μg/kg EB initially followed by 75 μg/kg dosing at predefined intervals as determined by clinical response. Twelve PERSEPT 3 subjects (2-56 years) received an initial preoperative infusion of 75 μg/kg (minor procedures) or 200 μg/kg EB (major surgeries) with subsequent 75 μg/kg doses administered intraoperatively and post-operatively as indicated. Descriptive statistics were used for data analyses.
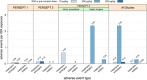
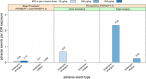
Results: Sixty subjects who received 3388 EB doses in three trials were evaluated. EB was well tolerated, with no allergic, hypersensitivity, anaphylactic or thrombotic events reported and no neutralizing anti-EB antibodies detected. A death occurred during PERSEPT 3 and was determined to be unlikely related to EB treatment by the data monitoring committee.
Conclusion: Results from all three Phase 3 trials establish an excellent safety profile of EB in haemophilia A or B patients with inhibitors for treatment of bleeding and perioperative use.
Keywords: PERSEPT; SEVENFACT; eptacog beta; haemophilia; inhibitors; recombinant FVIIa; safety.
© 2021 The Authors. Haemophilia published by John Wiley & Sons Ltd.
Conflict of interest statement
M.E. has received honoraria for consulting and participating in advisory boards from BioMarin, Novo Nordisk, Genentech, Sanofi, Takeda, Pfizer, Kedrion, CSL Behring and NHF. G.C. has received honoraria as a speaker/participant on advisory boards for Alexion, Bayer, Shire/Takeda, CSL Behring, Novo Nordisk, Sobi, Roche, uniQure, Sanofi, Werfen, Kedrion, LFB and Grifols; M.C. has received grant/research support from Roche/Genentech; has participated in speaker's bureaus with Takeda, Genentech, Bayer and Novo Nordisk; has received honoraria from Takeda, Roche/Genentech, Pfizer, HEMA Biologics, Spark, BioMarin, Sanofi, Kedrion, Grifols and Bayer; is a major stock shareholder in Alnylum; and has served on advisory boards for Takeda, Roche/Genentech, Pfizer, HEMA Biologics, Spark, uniQure, BioMarin, Sanofi, Kedrion, Grifols and Bayer. P.d.M. has received consulting fees from Novo Nordisk, LFB and Bayer. J.D. has acted as a consultant for Bayer and HEMA Biologics, and has been on the speaker's bureau for Bayer. C.L. has received honoraria for advisory board participation for Bayer, Catalyst, CSL Behring, Genentech, Sanofi and Takeda. J.L. has acted as a paid consultant to Novo Nordisk. J.M. has received research grants from Bayer, Biogen, BioMarin, CSL, Novo Nordisk, Sobi, Roche and uniQure; has served on scientific advisory committees of Amgen, Bayer, Biotest, Biogen, Baxalta, CSL Behring, Catalyst Biosciences, Novo Nordisk, Roche and Spark; and has been a member of the speaker bureau of Alnylam, Bayer, Biotest, Biogen, Novo Nordisk, Pfizer, Sobi, Shire, Roche, ISTH and WFH. W.M. declares interests with Alnylam, Bayer, Biogen, Chugai, CSL Behring, Novo Nordisk, Octapharma, Pfizer, LFB, Roche, Takeda, Freeline, BioMarin, Sobi and uniQure. C.N. has received honoraria/consultation fees or grants/research support from Bayer, CSL Behring, Freeline, LFB, Novo Nordisk, Octapharma, Pfizer, Roche‐Chugai, Sanofi, Shire‐Takeda, Sobi and Spark Therapeutics. D.Q. had received honoraria/consulting fees from Bayer, BioMarin, Bioverativ/Sanofi, Catalyst, Novo Nordisk and Roche/Genentech; and has been on the speaker's bureau for BioMarin, Bioverativ/Sanofi, Novo Nordisk, Takeda, and Roche/Genentech. M.R.’s employers have received research funding from Bayer, BioMarin, CSL Behring, Genentech, Grifols, HEMA Biologics, LFB, Novo Nordisk, Octapharma, Pfizer, Sanofi, Spark, Takeda and uniQure. M.R. has acted as a paid consultant to Catalyst Biosciences, CSL Behring, Genentech, HEMA Biologics, Kedrion, Novo Nordisk, Pfizer, Sanofi, Takeda and uniQure. M.R. is on the board of directors of Foundation for Women and Girls with Blood Disorders and Partners in Bleeding Disorders. M.R. is an employee of the American Thrombosis and Hemostasis Network and Oregon Health & Science University. J.F.S has received grants from Pfizer, Bayer, Novo Nordisk and LFB, and has fees for consulting to Sobi. A.D.S. has served on advisory boards or as consultant for Genentech, Roche, Novo Nordisk, BioMarin, Bioverativ, Sanofi, ProMetric Bio Sciences, Sangamo, Sigilon and Takeda; and has received research funding from these organizations plus Agios, OPKO Global Bio Therapeutics, Kedrion, Octapharma and Novartis. R.F.S. has acted as a paid consultant for Takeda, Sanofi, Sobi, Catalyst, BioMarin, Novo Nordisk, Genentech, Octapharma, Bayer, Pfizer, Grifols, Kedrion and HEMA Biologics; and has investigator‐initiated grants from Grifols (Mexico Inhibitor Study), Takeda (ATHN 9), Genentech and Octapharma (Emi PUPs and Nuwiq ITI) and Octapharma (MOTIVATE study). A.S. has served as the chair of the DMC for this study. M.W. has been a consultant and/or advisor to Bioverativ/Sanofi, Takeda, CSL Behring, Catalyst Biosciences, Novo Nordisk, Bayer, Octapharma, Genentech, HEMA Biologics, BioMarin and uniQure, and was a study investigator for HEMA Biologics for research carried out in this work. G.Y. has received honoraria for consulting for Genentech/Roche and a grant from Genentech. G.Y. also has received honoraria from BioMarin, Grifols, Pfizer, Sanofi, Spark, and Takeda; and has grants from Grifols and Takeda. W.A.A. works as a consultant for HEMA Biologics, LLC, and has received fees for speaking and consulting. A.A‐S. and D.B. are employees of LFB‐USA. C.M. is an employee of HEMA Biologics, LLC. T.A.W. is a medical writer for GLOVAL LLC. C.K. received research support from Bayer, Genentech, Novo Nordisk, Octapharma and Takeda; and has served on advisory boards for Bayer, CSL, Genentech, Novo Nordisk, Octapharma, Takeda, Pfizer and HEMA Biologics. S.B.B., C.H., J.J., I.H.M., O.S. and K.V.V. have no competing interests to declare.
Figures
References
-
- Astermark J. Overview of inhibitors. Semin Hematol. 2006;43(2 Suppl 4):S3‐7. - PubMed
-
- Négrier C, Gomperts ED, Oldenburg J. The history of FEIBA: a lifetime of success in the treatment of haemophilia complicated by an inhibitor. Haemophilia. 2006;12(s5):4‐13.
-
- Hedner U. Recombinant activated factor VII: 30 years of research and innovation. Blood Rev. 2015;29 Suppl 1:S4‐8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

