Benefits and harms of sodium-glucose co-transporter-2 inhibitors (SGLT2-I) and renin-angiotensin-aldosterone system inhibitors (RAAS-I) versus SGLT2-Is alone in patients with type 2 diabetes: A systematic review and meta-analysis of randomized controlled trials
- PMID: 34636161
- PMCID: PMC8754244
- DOI: 10.1002/edm2.303
Benefits and harms of sodium-glucose co-transporter-2 inhibitors (SGLT2-I) and renin-angiotensin-aldosterone system inhibitors (RAAS-I) versus SGLT2-Is alone in patients with type 2 diabetes: A systematic review and meta-analysis of randomized controlled trials
Abstract
Introduction: It is uncertain if the combination of sodium-glucose co-transporter 2 inhibitors (SGLT2-Is) and renin-angiotensin-aldosterone system inhibitors (RAAS-Is) provides better cardio-renal clinical outcomes in people with type 2 diabetes mellitus (T2DM) compared with SGLT2-Is alone. Using a systematic review and meta-analysis of randomized controlled trials (RCTs), we evaluated the efficacy and safety with respect to cardio-renal outcomes of the combination of SGLT2 and RAAS inhibitors vs SGLT2-Is in patients with T2DM.
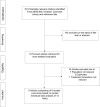
Methods: Studies were identified from MEDLINE, Embase, the Cochrane Library and search of bibliographies to May 2021. The Cochrane risk of bias tool was used to assess the risk of bias of each study. Study-specific risk ratios (RRs) with 95% confidence intervals (CIs) were pooled. Quality of the evidence was assessed using GRADE.
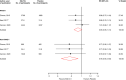
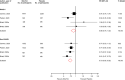
Results: Nine articles comprising 8 RCT evaluations (n = 34,551 participants) that compared SGLT2-Is with placebo in patients with T2DM against a background of standard care and reported subgroup results for those treated with or without RAAS-Is at baseline were included. No RCT specifically investigated the combination of SGLT2 and RAAS inhibitors compared with SGLT2-Is alone. The RRs (95% CIs) for composite cardiovascular outcome and composite CVD death/heart failure hospitalization comparing SGLT2-Is vs placebo in patients on RAAS-Is were 0.93 (0.85-1.01) and 0.88 (0.76-1.02), respectively. The corresponding estimates for patients not on RAAS-Is were 0.78 (0.65-0.93) and 0.73 (0.65-0.82), respectively. There was no evidence of interactions between RAAS-I status and the effects of SGLT2-Is for both outcomes. Single study results showed that SGLT2-Is vs placebo reduced the risk of composite kidney outcome and cardiovascular death in patients with RAAS inhibition. The effect of SGLT2 inhibition vs placebo on kidney parameters, genital infections, volume depletion, hyperkalaemia, hypokalaemia, hypoglycaemia and other adverse events was similar in patients with or without RAAS inhibition. The quality of the evidence ranged from very low to moderate.
Conclusions: Aggregate published data suggest that the combination of SGLT2 and RAAS inhibitors in the treatment of patients with T2DM may be similar in efficacy and safety if not superior to SGLT2-Is alone. Head-to-head comparisons of the two interventions are warranted to inform T2DM management. The use of SGLT2 inhibition as a first-line therapy in T2DM or its early use in the prevention of renal deterioration and cardiovascular complications in addition to its glycaemic control deserves further study.
Keywords: RAAS inhibitor; SGLT2 inhibitor; Type 2 diabetes.
© 2021 The Authors. Endocrinology, Diabetes & Metabolism published by John Wiley & Sons Ltd.
Conflict of interest statement
Dr. Khunti reports personal fees from Amgen, personal fees from Astrazeneca, personal fees from Bayer, personal fees from NAPP, personal fees from Lilly, personal fees from Merck Sharp & Dohme, personal fees from Novartis, personal fees from Novo Nordisk, personal fees from Roche, personal fees from Berlin‐Chemie AG / Menarini Group, personal fees from Sanofi‐Aventis, personal fees from Servier, personal fees from Boehringer Ingelheim, grants from Pfizer, grants from Boehringer Ingelheim, grants from AstraZeneca, grants from Novartis, grants from Novo Nordisk, grants from Sanofi‐Aventis, grants from Lilly, grants from Merck Sharp & Dohme, grants from Servier, outside the submitted work. Dr. Seidu reports personal fees from Amgen, personal fees from Astrazeneca, personal fees from NAPP, personal fees from Lilly, personal fees from Merck Sharp & Dohme, personal fees from Novartis, personal fees from Novo Nordisk, personal fees from Roche, personal fees from Sanofi‐Aventis, personal fees from Boehringer Ingelheim, grants from AstraZeneca, grants from Sanofi‐Aventis, grants from Servier, grants from Janssen, outside the submitted work. Dr Kunutor has no conflicts to report. Dr Topsever has received educational sponsorship or honoraria for speaking at meetings or serving at Advisory Boards from Boehringer Ingelheim, Lilly, Novo Nordisk and Sanofi Aventis.
Figures
References
-
- Disease GBD, Injury I, Prevalence C. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990‐2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1545‐1602. 2016/10/14. 10.1016/S0140-6736(16)31678-6 - DOI - PMC - PubMed
-
- Kramer H, Molitch ME. Screening for kidney disease in adults with diabetes. Diabetes Care. 2005;28:1813‐1816. - PubMed
-
- WHO Health Topics . Diabetes. https://www.who.int/health‐topics/diabetes#tab = tab_1. Accessed on 29 April 2021.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

