Reactivity of the Bicyclic Amido-Substituted Silicon(I) Ring Compound Si4 {N(SiMe3 )Mes}4 with FLP-Type Character
- PMID: 34636454
- PMCID: PMC9297995
- DOI: 10.1002/chem.202103101
Reactivity of the Bicyclic Amido-Substituted Silicon(I) Ring Compound Si4 {N(SiMe3 )Mes}4 with FLP-Type Character
Abstract
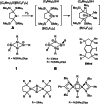
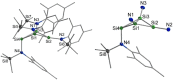
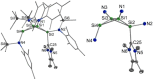
The bicyclic amido-substituted silicon(I) ring compound Si4 {N(SiMe3 )Mes}4 2 (Mes=Mesityl=2,4,6-Me3 C6 H2 ) features enhanced zwitterionic character and different reactivity from the analogous compound Si4 {N(SiMe3 )Dipp}4 1 (Dipp=2,6-i Pr2 C6 H3 ) due to the smaller mesityl substituents. In a reaction with the N-heterocyclic carbene NHC (1,3,4,5-tetramethyl-imidazol-2-ylidene), we observe adduct formation to give Si4 {N(SiMe3 )Mes}4 ⋅ NHC (3). This adduct reacts further with the Lewis acid BH3 to yield the Lewis acid-base complex Si4 {N(SiMe3 )Mes}4 ⋅ NHC ⋅ BH3 (4). Coordination of AlBr3 to 2 leads to the adduct 5. Calculated proton affinities and fluoride ion affinities reveal highly Lewis basic and very weak Lewis acidic character of the low-valent silicon atoms in 1 and 2. This is confirmed by protonation of 1 and 2 with Brookharts acid yielding 6 and 7. Reaction with diphenylacetylene only occurs at 111 °C with 2 in toluene and is accompanied by fragmentation of 2 to afford the silacyclopropene 8 and the trisilanorbornadiene species 9.
Keywords: N-heterocyclic carbenes; amines; frustrated Lewis pairs; inorganic ring systems; silicon.
© 2021 The Authors. Chemistry - A European Journal published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
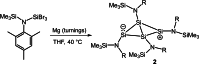










References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

