Chemoenzymatic Synthesis of Sialosides Containing 7- N- or 7,9-Di- N-acetyl Sialic Acid as Stable O-Acetyl Analogues for Probing Sialic Acid-Binding Proteins
- PMID: 34636559
- PMCID: PMC9242673
- DOI: 10.1021/acs.joc.1c01091
Chemoenzymatic Synthesis of Sialosides Containing 7- N- or 7,9-Di- N-acetyl Sialic Acid as Stable O-Acetyl Analogues for Probing Sialic Acid-Binding Proteins
Abstract
A novel chemoenzymatic synthon strategy has been developed to construct a comprehensive library of α2-3- and α2-6-linked sialosides containing 7-N- or 7,9-di-N-acetyl sialic acid, the stable analogue of naturally occurring 7-O-acetyl- or 7,9-di-O-acetyl-sialic acid. Diazido and triazido-mannose derivatives that were readily synthesized chemically from inexpensive galactose were shown to be effective chemoenzymatic synthons. Together with bacterial sialoside biosynthetic enzymes with remarkable substrate promiscuity, they were successfully used in one-pot multienzyme (OPME) sialylation systems for highly efficient synthesis of sialosides containing multiple azido groups. Conversion of the azido groups to N-acetyl groups generated the desired sialosides. The hydrophobic and UV-detectable benzyloxycarbonyl (Cbz) group introduced in the synthetic acceptors of sialyltransferases was used as a removable protecting group for the propylamine aglycon of the target sialosides. The resulting N-acetyl sialosides were novel stable probes for sialic acid-binding proteins such as plant lectin MAL II, which bond strongly to sialyl T antigens with or without an N-acetyl at C7 or at both C7 and C9 in the sialic acid.
Figures
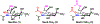
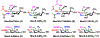


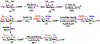
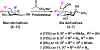
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

