Diastereocontrol in Radical Addition to β-Benzyloxy Hydrazones: Revised Approach to Tubuvaline and Synthesis of O-Benzyltubulysin V Benzyl Ester
- PMID: 34636574
- PMCID: PMC8576829
- DOI: 10.1021/acs.joc.1c01798
Diastereocontrol in Radical Addition to β-Benzyloxy Hydrazones: Revised Approach to Tubuvaline and Synthesis of O-Benzyltubulysin V Benzyl Ester
Abstract
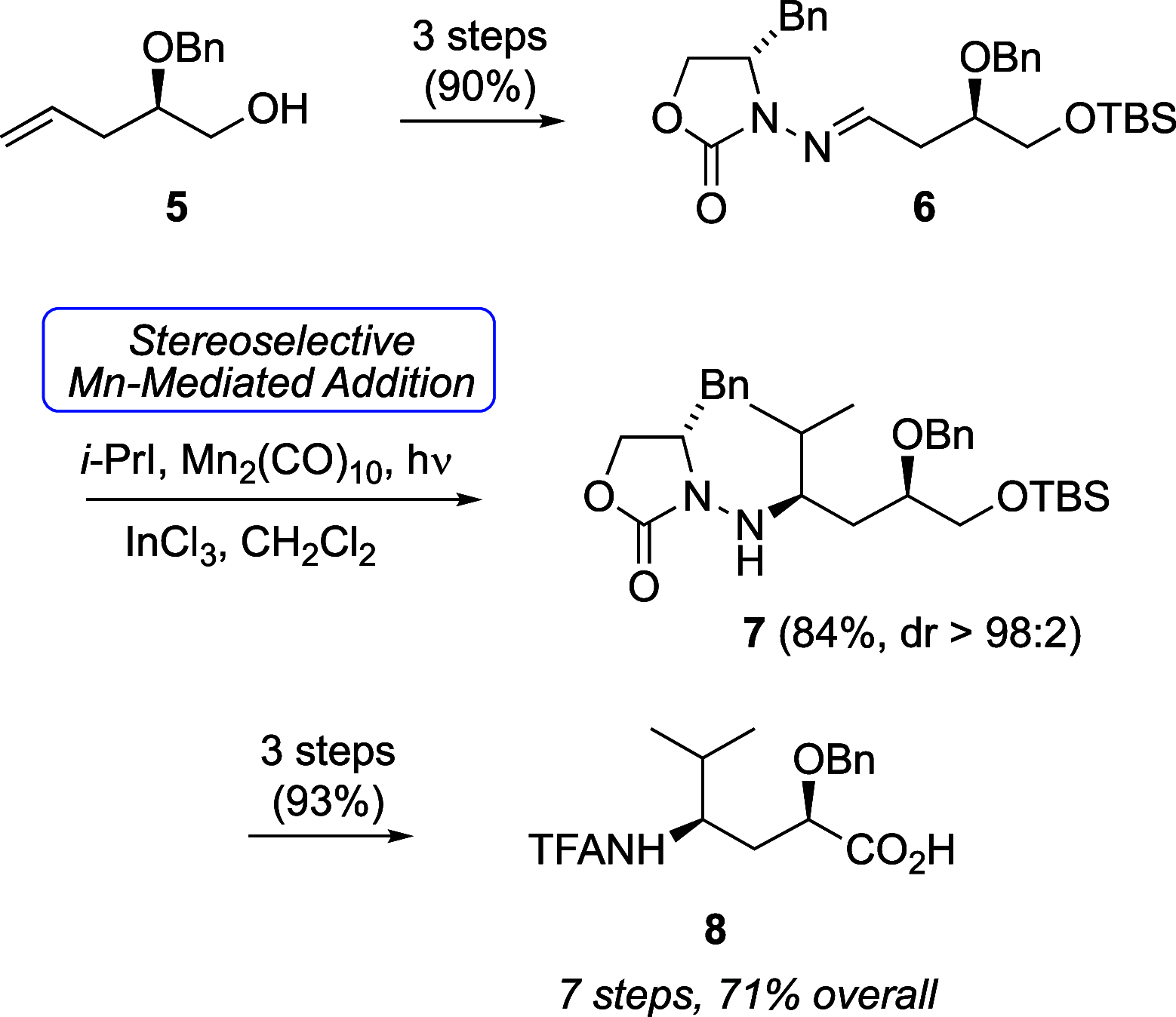
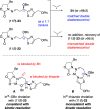
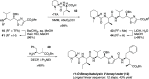
Radical addition to chiral N-acylhydrazones has generated unusual amino acids tubuphenylalanine (Tup) and tubuvaline (Tuv) that are structural components of the tubulysin family of picomolar antimitotic agents and previously led to a tubulysin tetrapeptide analog with a C-terminal alcohol. To improve efficiency in this synthetic route to tubulysins, and to address difficulties in oxidation of the C-terminal alcohol, here we present two alternative routes to Tuv that (a) improve step economy, (b) provide modified conditions for Mn-mediated radical addition in the presence of aromatic heterocycles, and (c) expose an example of double diastereocontrol in radical addition to a β-benzyloxyhydrazone with broader implications for asymmetric amine synthesis via radical addition. An efficient coupling sequence affords 11-O-benzyltubulysin V benzyl ester.
Conflict of interest statement
The authors declare no competing financial interest.
Figures







References
-
- Miyabe H.; Ushiro C.; Naito T. Highly diastereoselective radical addition to glyoxylic oxime ether: asymmetric synthesis of alpha-amino acids. Chem. Commun. 1997, 1789–1790. 10.1039/a704562j. - DOI - PubMed
- Miyabe H.; Ushiro C.; Ueda M.; Yamakawa K.; Naito T. Asymmetric synthesis of alpha-amino acids based on carbon radical addition to glyoxylic oxime ether. J. Org. Chem. 2000, 65, 176–185. 10.1021/jo991353n. - DOI - PubMed
-
- Bertrand M. P.; Coantic S.; Feray L.; Nouguier R.; Perfetti P. Et3B- and Et2Zn-mediated radical additions to glyoxylate imines, compared stereoinductions. Tetrahedron 2000, 56, 3951–3961. 10.1016/S0040-4020(00)00327-6. - DOI
- Bertrand M. P.; Feray L.; Nouguier R.; Perfetti P. Diethylzinc mediated radical additions to glyoxylate imines. Synlett 1999, 1999, 1148–1150. 10.1055/s-1999-2754. - DOI
- Bertrand M. P.; Feray L.; Nouguier R.; Stella L. 1,3-stereoinduction in radical additions to glyoxylate imines. Synlett 1998, 1998, 780–782. 10.1055/s-1998-1763. - DOI
-
- Friestad G. K.; Qin J. Highly Stereoselective Intermolecular Radical Addition to Aldehyde Hydrazones from a Chiral 3-Amino-2-oxazolidinone. J. Am. Chem. Soc. 2000, 122, 8329–8330. 10.1021/ja002173u. - DOI
-
- Friestad G. K.; Qin J.; Suh Y.; Marie J.-C. Mn-Mediated Coupling of Alkyl Iodides and Chiral N-Acylhydrazones: Optimization, Scope, and Evidence for a Radical Mechanism. J. Org. Chem. 2006, 71, 7016–7027. 10.1021/jo061158q. - DOI - PubMed
- Friestad G. K.; Qin J. Intermolecular Alkyl Radical Addition to Chiral N-Acylhydrazones Mediated by Manganese Carbonyl. J. Am. Chem. Soc. 2001, 123, 9922–9923. 10.1021/ja011312k. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

