Metagenomic Sequencing of Multiple Soil Horizons and Sites in Close Vicinity Revealed Novel Secondary Metabolite Diversity
- PMID: 34636675
- PMCID: PMC8510542
- DOI: 10.1128/mSystems.01018-21
Metagenomic Sequencing of Multiple Soil Horizons and Sites in Close Vicinity Revealed Novel Secondary Metabolite Diversity
Abstract
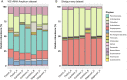
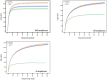
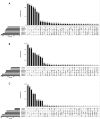
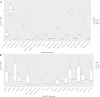
Discovery of novel antibiotics is crucial for combating rapidly spreading antimicrobial resistance and new infectious diseases. Most of the clinically used antibiotics are natural products-secondary metabolites produced by soil microbes that can be cultured in the lab. Rediscovery of these secondary metabolites during discovery expeditions costs both time and resources. Metagenomics approaches can overcome this challenge by capturing both culturable and unculturable hidden microbial diversity. To be effective, such an approach should address questions like the following. Which sequencing method is better at capturing the microbial diversity and biosynthesis potential? What part of the soil should be sampled? Can patterns and correlations from such big-data explorations guide future novel natural product discovery surveys? Here, we address these questions by a paired amplicon and shotgun metagenomic sequencing survey of samples from soil horizons of multiple forest sites very close to each other. Metagenome mining identified numerous novel biosynthetic gene clusters (BGCs) and enzymatic domain sequences. Hybrid assembly of both long reads and short reads improved the metagenomic assembly and resulted in better BGC annotations. A higher percentage of novel domains was recovered from shotgun metagenome data sets than from amplicon data sets. Overall, in addition to revealing the biosynthetic potential of soil microbes, our results suggest the importance of sampling not only different soils but also their horizons to capture microbial and biosynthetic diversity and highlight the merits of metagenome sequencing methods. IMPORTANCE This study helped uncover the biosynthesis potential of forest soils via exploration of shotgun metagenome and amplicon sequencing methods and showed that both methods are needed to expose the full microbial diversity in soil. Based on our metagenome mining results, we suggest revising the historical strategy of sampling soils from far-flung places, as we found a significant number of novel and diverse BGCs and domains even in different soils that are very close to each other. Furthermore, sampling of different soil horizons can reveal the additional diversity that often remains hidden and is mainly caused by differences in environmental key parameters such as soil pH and nutrient content. This paired metagenomic survey identified diversity patterns and correlations, a step toward developing a rational approach for future natural product discovery surveys.
Keywords: Oxford Nanopore; amplicon sequencing; biosynthetic gene clusters; metagenome; natural products; secondary metabolites; soil horizons.
Conflict of interest statement
We declare that we have no competing interests.
Figures

Similar articles
-
A rapid and efficient strategy to identify and recover biosynthetic gene clusters from soil metagenomes.Appl Microbiol Biotechnol. 2022 Apr;106(8):3293-3306. doi: 10.1007/s00253-022-11917-y. Epub 2022 Apr 18. Appl Microbiol Biotechnol. 2022. PMID: 35435454 Free PMC article.
-
Mining metagenomic data to gain a new insight into the gut microbial biosynthetic potential in placental mammals.Microbiol Spectr. 2024 Oct 3;12(10):e0086424. doi: 10.1128/spectrum.00864-24. Epub 2024 Aug 20. Microbiol Spectr. 2024. PMID: 39162518 Free PMC article.
-
Long-Read Metagenomics of Marine Microbes Reveals Diversely Expressed Secondary Metabolites.Microbiol Spectr. 2023 Aug 17;11(4):e0150123. doi: 10.1128/spectrum.01501-23. Epub 2023 Jul 6. Microbiol Spectr. 2023. PMID: 37409950 Free PMC article.
-
Culture-independent discovery of natural products from soil metagenomes.J Ind Microbiol Biotechnol. 2016 Mar;43(2-3):129-41. doi: 10.1007/s10295-015-1706-6. Epub 2015 Nov 19. J Ind Microbiol Biotechnol. 2016. PMID: 26586404 Review.
-
Application of computational approaches to analyze metagenomic data.J Microbiol. 2021 Mar;59(3):233-241. doi: 10.1007/s12275-021-0632-8. Epub 2021 Feb 10. J Microbiol. 2021. PMID: 33565054 Review.
Cited by
-
Uncovering Genomic Features and Biosynthetic Gene Clusters in Endophytic Bacteria from Roots of the Medicinal Plant Alkanna tinctoria Tausch as a Strategy To Identify Novel Biocontrol Bacteria.Microbiol Spectr. 2023 Aug 17;11(4):e0074723. doi: 10.1128/spectrum.00747-23. Epub 2023 Jul 12. Microbiol Spectr. 2023. PMID: 37436171 Free PMC article.
-
Uncovering encrypted antimicrobial peptides in health-associated Lactobacillaceae by large-scale genomics and machine learning.Microbiome. 2025 Jun 21;13(1):151. doi: 10.1186/s40168-025-02145-3. Microbiome. 2025. PMID: 40544303 Free PMC article.
-
Unlocking the biosynthetic potential and taxonomy of the Antarctic microbiome along temporal and spatial gradients.Microbiol Spectr. 2024 Jun 4;12(6):e0024424. doi: 10.1128/spectrum.00244-24. Epub 2024 May 15. Microbiol Spectr. 2024. PMID: 38747631 Free PMC article.
-
Diversity and distribution of biosynthetic gene clusters in agricultural soil microbiomes.mSystems. 2024 Apr 16;9(4):e0126323. doi: 10.1128/msystems.01263-23. Epub 2024 Mar 12. mSystems. 2024. PMID: 38470142 Free PMC article.
-
Deciphering the biosynthetic landscape of biofilms in glacier-fed streams.mSystems. 2025 Feb 18;10(2):e0113724. doi: 10.1128/msystems.01137-24. Epub 2024 Dec 31. mSystems. 2025. PMID: 39745394 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
