Glycan characteristics of human heart constituent cells maintaining organ function: relatively stable glycan profiles in cellular senescence
- PMID: 34637040
- PMCID: PMC8566412
- DOI: 10.1007/s10522-021-09940-z
Glycan characteristics of human heart constituent cells maintaining organ function: relatively stable glycan profiles in cellular senescence
Abstract
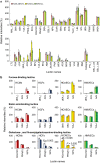
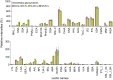
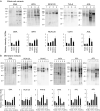
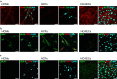
Cell surface glycoproteins, which are good indicators of cellular types and biological function; are suited for cell evaluation. Tissue remodeling using various cells is a key feature of regenerative therapy. For artificial heart remodeling, a mixture of heart constituent cells has been investigated for organ assembly, however, the cellular characteristics remain unclear. In this study, the glycan profiles of human cardiomyocytes (HCMs), human cardiac fibroblasts (HCFs), and human vascular endothelial cells (ECs) were analyzed using evanescent-field lectin microarray analysis, a tool of glycan profiling, to clarify the required cellular characteristics. We found that ECs had more "α1-2fucose" and "core α1-6fucose" residues than other cells, and that "α2-6sialic acid" residue was more abundant in ECs and HCMs than in HCFs. HCFs showed higher abundance of "β-galactose" and "β-N-acetylgalactosamine" residues on N-glycan and O-glycan, respectively, compared to other cells. Interestingly, cardiac glycan profiles were insignificantly changed with cellular senescence. The residues identified in this study may participate in organ maintenance by contributing to the preservation of glycan components. Therefore, future studies should investigate the roles of glycans in optimal tissue remodeling since identifying cellular characteristics is important for the development of regenerative therapies.
Keywords: Cardiac fibroblast; Cardiomyocyte; Cell surface protein; Glycan profile; Lectin microarray; Vascular endothelial cell.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
N- and O-glycan cell surface protein modifications associated with cellular senescence and human aging.Cell Biosci. 2016 Feb 18;6:14. doi: 10.1186/s13578-016-0079-5. eCollection 2016. Cell Biosci. 2016. PMID: 26893823 Free PMC article.
-
Qualitative and quantitative alterations in intracellular and membrane glycoproteins maintain the balance between cellular senescence and human aging.Aging (Albany NY). 2018 Aug 29;10(8):2190-2208. doi: 10.18632/aging.101540. Aging (Albany NY). 2018. PMID: 30157474 Free PMC article.
-
[Alteration of the glycan profile of serum glycoproteins during the seroconversion process in hepatitis B virus-infected patients treated with antiviral therapy and its clinical significance].Zhonghua Gan Zang Bing Za Zhi. 2014 Sep;22(9):660-6. doi: 10.3760/cma.j.issn.1007-3418.2014.09.006. Zhonghua Gan Zang Bing Za Zhi. 2014. PMID: 25369249 Chinese.
-
Glycan Labeling and Analysis in Cells and In Vivo.Annu Rev Anal Chem (Palo Alto Calif). 2021 Jul 27;14(1):363-387. doi: 10.1146/annurev-anchem-091620-091314. Annu Rev Anal Chem (Palo Alto Calif). 2021. PMID: 34314224 Review.
-
In Situ Cellular Glycan Analysis.Acc Chem Res. 2018 Apr 17;51(4):890-899. doi: 10.1021/acs.accounts.7b00617. Epub 2018 Mar 29. Acc Chem Res. 2018. PMID: 29595964 Review.
Cited by
-
Spatiotemporal changes of tissue glycans depending on localization in cardiac aging.Regen Ther. 2023 Jan 7;22:68-78. doi: 10.1016/j.reth.2022.12.009. eCollection 2023 Mar. Regen Ther. 2023. PMID: 36712959 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

